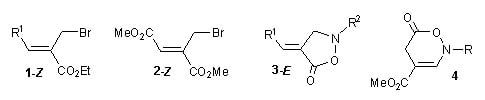An Expeditious Synthesis of [1,2]Isoxazolidin-5-ones and [1,2]Oxazin-6-ones from Functional Allyl Bromide Derivatives
Abstract
:1. Introduction

2. Results and Discussion

| Product | R1 | R2 | Yield a (%) |
|---|---|---|---|
| 3a | nC3H7 | tC4H9 | 60 |
| 3b | C6H5 | tC4H9 | 83 |
| 3c | nC3H7 | cC6H11 | 48 |
| 3d | C6H5 | cC6H11 | 61 |
| 3e | nC5H11 | cC6H11 | 56 |

| Product | R | Yield a (%) |
|---|---|---|
| 4a | iC3H7 | 79 |
| 4b | tC4H9 | 76 |
| 4c | cC6H11 | 58 |
| 4d | C6H5 | 35 |
| 4e | CH2C6H5 | 6 |
3. Experimental
3.1. General
3.2. General procedure for the synthesis of (E)-4-alkylidene-2-alkylisoxazolidin-5-ones 3
3.3. General procedure for the synthesis of methyl 2-alkyl-6-oxo-5,6-dihydro-2H-1,2-oxazine-4-carboxylates 4
4. Conclusions
- Sample Availability: Contact the authors.
References
- Mattes, H.; Benezra, C. Reformatsky-type reactions in aqueous media. Use of bronometryl-acrylic acid for the synthesis of α-methylene-γ-butyrolactones. Tetrahedron Lett. 1985, 26, 5697–5698. [Google Scholar] [CrossRef]
- Fürstner, A. Recent advancements in the Reformatsky reaction. Synthesis 1989, 571–590. [Google Scholar] [CrossRef]
- Hoffmann, H.M.R.; Rabe, J. Synthesis and biological activity of α-methylene-γ-butyrolactones. Angew.Chem. Int. Ed. Engl. 1985, 24, 94–110. [Google Scholar] [CrossRef]
- Petragnani, N.; Ferraz, H.M.C.; Silva, G.V.J. Advances in the synthesis of α-methylenelactones. Synthesis 1986, 157–183. [Google Scholar]
- Baldwin, J.E.; Adlington, R.M.; Sweeney, J.B. Improved synthesis of α-methylene-γ-lactones via organotin reagents. Tetrahedron Lett. 1986, 27, 5423–5424. [Google Scholar] [CrossRef]
- El Alami, N.; Belaud, C.; Villiéras, J. Isolement du “reactif de Reformatsky” derive de l'α-(bromomethyl) acrylate d'ethyle. Tetrahedron Lett. 1987, 28, 59–60. [Google Scholar] [CrossRef]
- El Alami, N.; Belaud, C.; Villiéras, J. La reaction de “Reformatsky” de l'α-(bromomethyl) acrylate d'ethyle avec les chlorures d'acide et les nitriles. J. Organomet. Chem. 1987, 319, 303–309. [Google Scholar]
- Besbes, R.; Villiéras, M.; Amri, H. Improved synthesis and reaction of dimethyl α-(bromomethyl) fumarate with primary amines. Indian J. Chem. 1997, 36B, 5–8. [Google Scholar]
- Basavaiah, D.; Rao, J.S. Applications of Baylis-Hillman acetates: one-pot, facile and convenient synthesis of substituted γ-lactams. Tetrahedron Lett. 2004, 45, 1621–1625. [Google Scholar] [CrossRef]
- Ferris, A.F. The action of mineral acid on diethyl bis(hydroxymethyl)malonate. J. Org. Chem. 1955, 20, 780–787. [Google Scholar]
- Villiéras, J.; Rambaud, M. Ethyl α-(hydroxymethyl)acrylate. Org. Synth. 1988, 66, 220–224. [Google Scholar]
- Villiéras, J.; Rambaud, M. Wittig-Horner reaction in heterogeneous media; 1. An easy synthesis of ethyl α-hydroxymethylacrylate and ethyl α-halomethylacrylates using formaldehyde in water. Synthesis 1982, 924–926. [Google Scholar]
- Charlton, J.L.; Sayeed, V.A.; Lypka, G.N. Phase transfer catalysed synthesis of methyl α-bromomethylacrylate. Synth. Commun. 1981, 11, 931–934. [Google Scholar] [CrossRef]
- Holm, A.; Scheuer, P.J. Synthesis of α-methylene-β-alanine and one of its naturally occurring α-ketomides. Tetrahedron Lett. 1980, 21, 1125–1128. [Google Scholar] [CrossRef]
- Arfaoui, A.; Amri, H. An effective new access to ethyl 2-[(alkylamino)(cyano)methyl] acrylates: First synthesis of ethyl 3-cyano-2-(hydroxymethyl) acrylate. Tetrahedron 2009, 65, 4904–4907. [Google Scholar] [CrossRef]
- Beltaief, I.; Hbaïeb, S.; Besbes, R.; Villiéras, M.; Villiéras, J.; Amri, H. A new and efficient method for the isomerization of secondary functional allylic alcohols into their primary isomers. Synthesis 1998, 1765–1768. [Google Scholar]
- Lee, M.J.; Kim, S.C.; Kim, J.N. The first synthesis of 3,5-dimethylene-4-phenylpiperidine-2,6- dione from Baylis-Hillman adduct. Bull. Korean Chem. Soc. 2006, 27, 140–142. [Google Scholar] [CrossRef]
- Lee, K.Y.; Kim, S.C.; Kim, J.N. Regioselective synthesis of 1-arylnaphthalenes from N-tosylaziridine derivatives. Tetrahedron Lett. 2006, 47, 977–980. [Google Scholar] [CrossRef]
- Lee, K.Y.; Seo, J.; Kim, J.N. Serendipitous synthesis of 2-amino-2,3-dihydrobenzofuran derivatives starting from Baylis-Hillman adducts. Tetrahedron Lett. 2006, 47, 3913–3917. [Google Scholar] [CrossRef]
- Vaughan, W.R.; Milton, K.M. α-Bromocitraconic anhydride and α-bromomesaconic acid. J. Am. Chem. Soc. 1951, 73, 5497–5498. [Google Scholar] [CrossRef]
- Beltaief, I.; Besbes, R.; Ben Amor, F.; Villiéras, M.; Villiéras, J.; Amri, H. (Z)-Dimethyl α-(bromomethyl) fumarate, an efficient intermediate for the selective synthesis of dimethyl 3- alkyl itaconates and 2-alkyl 3-carbomethoxy-γ-lactams. Tetrahedron 1999, 55, 3949–3958. [Google Scholar] [CrossRef]
- Beji, F.; Lebreton, J.; Villiéras, J.; Amri, H. A total stereospecific route to α-alkylidene-γ- lactams. Tetrahedron 2001, 57, 9959–9962. [Google Scholar] [CrossRef]
- Beji, F.; Besbes, R.; Amri, H. Synthesis of α-alkylidene-β-ethoxycarbonyl cyclopentanones and -γ-butyrolactones. Synth. Commun. 2000, 30, 3947–3954. [Google Scholar]
- Stamm, H.; Steudle, H. Nitrone-XI Isoxazolidin-verbindungen-VIII: N-substituierte 5-isoxazolidinone durch reformatzky-reaktion mit nitronen. Tetrahedron 1979, 35, 647–650. [Google Scholar] [CrossRef]
- Lee, K.Y.; Lee, C.G.; Kim, T.H.; Kim, J.N. A facile synthesis of 4-arylidene-2-substituted Isoxazolidin-5-ones from Baylis-Hillman acetates. Bull. Korean Chem. Soc. 2004, 25, 33–34. [Google Scholar] [CrossRef]
- Stamm, H.; Steudle, H. The mechanism of hydroxylamine addition to α,β-unsaturated esters. Tetrahedron Lett. 1976, 17, 3607–3610. [Google Scholar] [CrossRef]
- Sibi, M.P.; Prabagaran, N.; Ghorpade, S.G.; Jasperse, C.P. Enantioselective synthesis of α,β-disubstituted-β-amino acids. J. Am. Chem. Soc. 2003, 125, 11796–11797. [Google Scholar] [CrossRef]
- Sibi, M.P.; Liu, M. N-Benzylhydroxylamine addition to β-aryl enoates. Enantioselective Synthesis of β-aryl-β-amino acid precursors. Org. Lett. 2000, 2, 3393–3396. [Google Scholar] [CrossRef]
- Ishikawa, T.; Nagai, K.; Senzaki, M.; Tatsukawa, A.; Saito, S. Tetrahedron 1998, 54, 2433–2448.
- Sibi, M.P.; Liu, M. Enantioselective conjugate addition of hydroxylamines to pyrazolidinone acrylamides. Org. Lett. 2001, 3, 4181–4184. [Google Scholar] [CrossRef]
- Socha, D.; Jurczak, M.; Chmielewski, M. Synthesis of acosamine and daunosamine from sugar δ-enelactones. Tetrahedron 1997, 53, 739–746. [Google Scholar] [CrossRef]
- Panfil, I.; Maciejewski, S; Bezecki, C.; Chmielewski, M. Synthesis of enantiomerically pure 2,3-disubstituted isoxazolidin-5-ones. Tetrahedron Lett. 1989, 30, 1527–1528. [Google Scholar]
- Socha, D.; Jurczak, M.; Chmielewski, M. Stereocontrolled entry to Negamycin from D-Glucose. Tetrahedron Lett. 1995, 36, 135–138. [Google Scholar] [CrossRef]
- Maciejewski, S; Panfil, I.; Bezecki, C.; Chmielewski, M. An approach to carbapenems from α,β- unsaturated sugar lactones. Tetrahedron 1992, 48, 10363–10376. [Google Scholar] [CrossRef]
- Baldwin, S.W.; Aubé, J. Asymmetric synthesis with chiral hydroxylamines: Synthesis of optically pure 4-substituted azetidinones. Tetrahedron Lett. 1987, 28, 179–182. [Google Scholar] [CrossRef]
- Merino, P.; Franco, S.; Merchan, F.L.; Tejero, T. Synthesis of isoxazolidin-5-ones via stereocontrolled Michael additions of benzylhydroxylaminen to L-serine derived α,β-unsaturated esters. Tetrahedron Asymmetry 1998, 9, 3945–3949. [Google Scholar] [CrossRef]
- Hbaieb, S.; Latiri, Z.; Amri, H. Stereoselective synthesis of 2-ethylidene-3-aminonitriles. Synth. Commun. 1999, 29, 981–988. [Google Scholar] [CrossRef]
- Terano, H.; Takase, S.; Hosoda, J.; Kohsaka, M. A new antitumor antibiotic, FR-66979. J. Antibiot. 1989, 42, 145–148. [Google Scholar] [CrossRef]
- Naoe, Y.; Inami, M.; Matsumoto, S.; Nishigaki, F.; Tsujimoto, S.; Kawamura, I.; Miyayasu, K.; Manda, T.; Shimomura, K. FK317: A novel substituted dihydrobenzoxazine with potent antitumor activity which does not induce vascular leak syndrome. Cancer Chemother. Pharmacol. 1998, 42, 31–36. [Google Scholar] [CrossRef]
- Inami, M.; Kawamura, I.; Tsujimoto, S.; Nishigaki, F.; Matsumoto, S.; Naoe, Y.; Sasakawa, Y.; Matsuo, M.; Manda, T.; Goto, T. Effects of FK317, a novel anti-cancer agent, on survival of mice bearing B16BL6 melanoma and Lewis lung carcinoma. Cancer Lett. 2002, 181, 39–45. [Google Scholar] [CrossRef]
- Miyata, O.; Namba, M.; Ueda, M.; Naito, T. A novel synthesis of amino-1,2-oxazinones as a versatile synthon for β-amino acid derivatives. Org. Biomol. Chem. 2004, 2, 1274–1276. [Google Scholar] [CrossRef]
- Le Flohic, A.; Meyer, C.; Cossy, J.; Desmurs, J.R. Synthesis of unsaturated [1,2]oxazines by using sigmatropic rearrangements and the ring-closing metathesis reaction. Tetrahedron Lett. 2003, 44, 8577–8580. [Google Scholar]
- Yang, Y.-K.; Tae, J. Ring-Closing Metathesis of enynes tethered by an N-O Bond: Synthesis of 1,2-oxaza polycycles by Diels-Alder reaction of the Ring-Closing Metathesis products. Synlett 2003, 2017–2020. [Google Scholar]
- Koide, K.; Finkelstein, J.M.; Ball, Z.; Verdine, G.L. A synthetic library of cell-permeable molecules. J. Am. Chem. Soc. 2001, 123, 398–408. [Google Scholar]
- Reddy, V.K.; Miyabe, H.; Yamauchi, M.; Takemoto, Y. Enantioselective synthesis of [1,2]-oxazinone scaffolds and [1,2]-oxazine core structures of FR900482. Tetrahedron 2008, 64, 1040–1048. [Google Scholar] [CrossRef]
- Patel, S.K.; Murat, K.; Py, S.; Vallée, Y. Asymmetric total synthesis and stereochemical elucidation of the antitumor agent PM-94128. Org. Lett. 2003, 5, 4081–4084. [Google Scholar] [CrossRef]
- Le Flohic, A.; Meyer, C.; Cossy, J.; Desmurs, J.R. Synthesis of unsaturated [1,2]oxazines by using sigmatropicrearrangements and the ring-closing metathesis reaction. Tetrahedron Lett. 2003, 44, 8577–8580. [Google Scholar]
- Baldwin, J.E.; Cutting, J.; Dupont, J.; Kruse, L.; Silberman, L.; Thomas, R.C. 5-Endo-trigonal reactions: A disfavoured ring closure. J. Chem. Soc. Chem. Commun. 1976, 736–738. [Google Scholar]
© 2010 by the authors;
Share and Cite
Beltaïef, I.; Arfaoui, A.; Amri, H. An Expeditious Synthesis of [1,2]Isoxazolidin-5-ones and [1,2]Oxazin-6-ones from Functional Allyl Bromide Derivatives. Molecules 2010, 15, 4094-4101. https://doi.org/10.3390/molecules15064094
Beltaïef I, Arfaoui A, Amri H. An Expeditious Synthesis of [1,2]Isoxazolidin-5-ones and [1,2]Oxazin-6-ones from Functional Allyl Bromide Derivatives. Molecules. 2010; 15(6):4094-4101. https://doi.org/10.3390/molecules15064094
Chicago/Turabian StyleBeltaïef, Imen, Aïcha Arfaoui, and Hassen Amri. 2010. "An Expeditious Synthesis of [1,2]Isoxazolidin-5-ones and [1,2]Oxazin-6-ones from Functional Allyl Bromide Derivatives" Molecules 15, no. 6: 4094-4101. https://doi.org/10.3390/molecules15064094