Evaluation of Carbohydrates in Natural and Cultured Cordyceps by Pressurized Liquid Extraction and Gas Chromatography Coupled with Mass Spectrometry
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of Derivatization

2.2. Optimization of Trifluoroacetic Acid (TFA) Hydrolysis

2.3. Optimization of Pressurized Liquid Extraction (PLE)
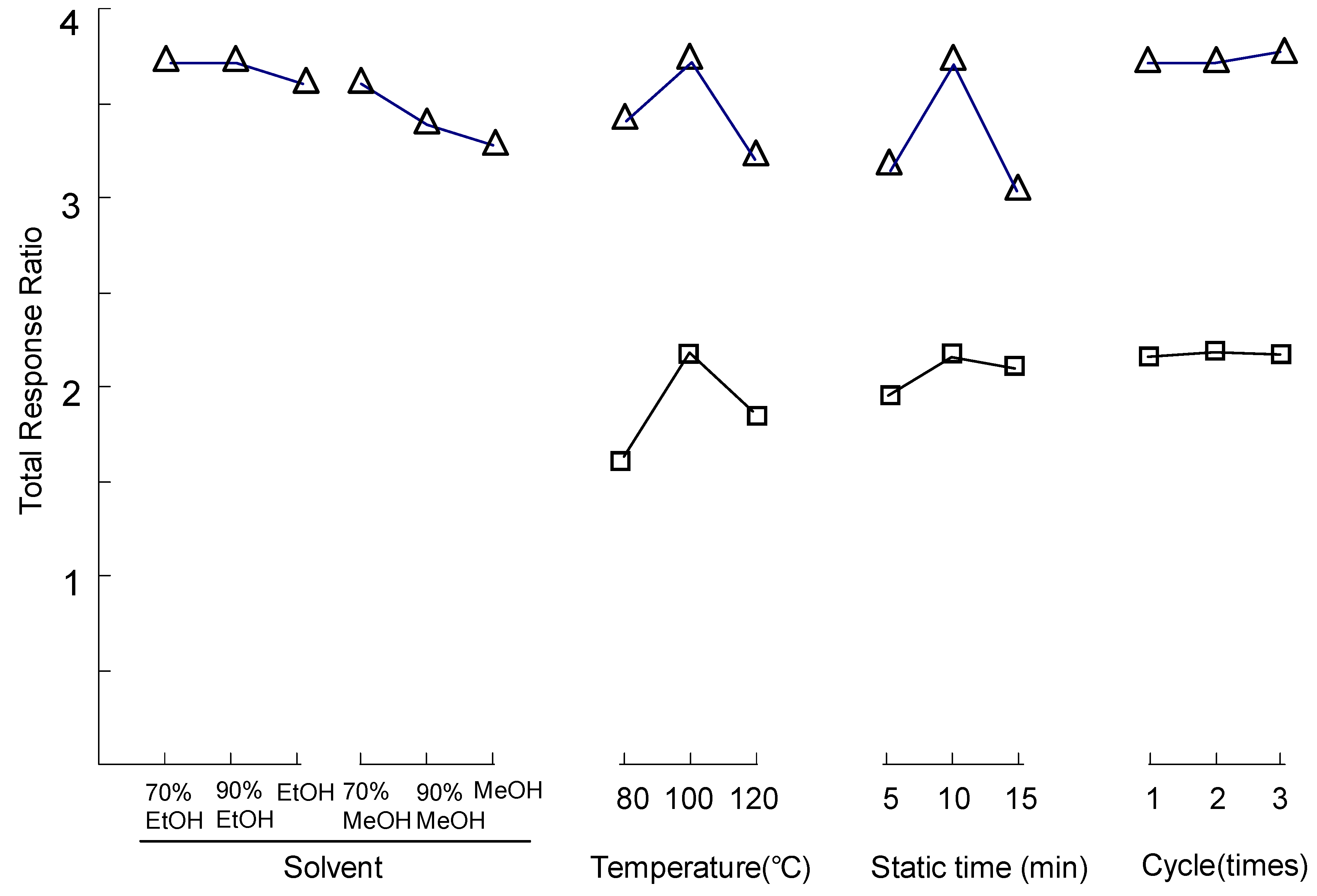
2.4. Method Validation
| Analytes | SIM ( m/z) | Linear regression data | LOQ (ng) | LOD (ng) | ||
|---|---|---|---|---|---|---|
| Regression equation | R2 | Test range (ng) | ||||
| Rhamnose | 129 | y = 0.0407x - 0.0026 | 0.9996 | 0.96-31.94 | 0.33 | 0.08 |
| Ribose | 115 | y = 0.0476x + 0.0283 | 0.9908 | 0.97-32.38 | 0.16 | 0.08 |
| Arabinose | 115 | y = 0.0405x - 0.0025 | 0.9993 | 0.97-32.28 | 0.17 | 0.08 |
| Xylose | 115 | y = 0.0421x - 0.0015 | 0.9995 | 0.96-32.12 | 0.45 | 0.08 |
| Mannose | 115 | y = 0.0402x - 0.0068 | 0.9996 | 0.97-32.41 | 0.16 | 0.08 |
| Glucose | 115 | y = 0.0361x - 0.0046 | 0.9997 | 0.96-32.04 | 0.16 | 0.13 |
| Galactose | 115 | y = 0.0361x - 0.0095 | 0.9993 | 0.98-32.52 | 0.33 | 0.17 |
| Mannitol | 115 | y = 0.0746x + 0.0038 | 0.9996 | 1.93-64.28 | 0.25 | 0.12 |
| Fructose | 345 | y = 0.0512x - 0.1583 | 0.9820 | 3.26-32.65 | 1.60 | 0.98 |
| Sorbose | 345 | y = 0.0598x - 0.1297 | 0.9927 | 1.61-32.18 | 1.29 | 0.88 |
| Analyte | Contain (ng) | Short term (n = 6) | Long term (n = 6) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Found (ng) | Accuracy(%) a | RSD (%) | Found (ng) | Accuracy(%) | RSD (%) | |||||
| RtRb | RPac | RtR | RPa | |||||||
| Rhamnose | 3.19 | 3.11 | 97.45 | 0.01 | 1.22 | 3.11 | 97.56 | 0.07 | 1.55 | |
| 15.97 | 15.65 | 98.17 | 0.02 | 1.40 | 15.55 | 97.58 | 0.06 | 1.38 | ||
| 25.55 | 25.12 | 98.52 | 0.05 | 1.04 | 25.25 | 99.01 | 0.09 | 1.21 | ||
| Ribose | 3.24 | 2.82 | 87.38 | 0.02 | 0.97 | 3.11 | 88.36 | 0.06 | 1.27 | |
| 16.19 | 16.67 | 103.15 | 0.02 | 0.72 | 16.46 | 103.15 | 0.06 | 5.19 | ||
| 25.91 | 24.28 | 93.89 | 0.05 | 1.11 | 24.27 | 93.88 | 0.11 | 1.03 | ||
| Arabinose | 3.23 | 3.12 | 96.86 | 0.01 | 0.79 | 3.13 | 97.14 | 0.07 | 1.07 | |
| 16.14 | 15.58 | 96.70 | 0.02 | 0.93 | 15.56 | 96.58 | 0.05 | 1.65 | ||
| 25.82 | 25.58 | 99.21 | 0.04 | 0.83 | 25.71 | 99.74 | 0.10 | 0.62 | ||
| Xylose | 3.21 | 3.16 | 98.53 | 0.02 | 1.59 | 3.17 | 98.90 | 0.07 | 1.49 | |
| 16.06 | 15.68 | 97.79 | 0.04 | 0.84 | 15.61 | 97.42 | 0.07 | 1.05 | ||
| 25.69 | 25.45 | 99.23 | 0.05 | 0.78 | 25.63 | 99.94 | 0.11 | 1.13 | ||
| Mannose | 3.24 | 3.13 | 96.86 | 0.01 | 1.42 | 3.17 | 97.92 | 0.06 | 1.52 | |
| 16.20 | 15.89 | 98.26 | 0.01 | 0.61 | 15.83 | 97.85 | 0.06 | 0.94 | ||
| 25.93 | 25.58 | 98.84 | 0.03 | 0.95 | 25.63 | 99.04 | 0.07 | 0.47 | ||
| Glucose | 3.20 | 3.05 | 95.26 | 0.01 | 0.87 | 3.07 | 96.10 | 0.06 | 1.89 | |
| 16.02 | 15.77 | 98.64 | 0.03 | 0.80 | 15.70 | 98.18 | 0.05 | 1.91 | ||
| 25.63 | 25.40 | 99.26 | 0.03 | 0.74 | 25.38 | 99.19 | 0.08 | 0.34 | ||
| Galactose | 3.25 | 3.06 | 94.38 | 0.01 | 1.13 | 3.08 | 95.01 | 0.06 | 1.46 | |
| 16.26 | 15.71 | 96.80 | 0.03 | 1.89 | 15.84 | 97.56 | 0.06 | 1.69 | ||
| 26.02 | 25.85 | 99.54 | 0.03 | 1.05 | 25.93 | 99.84 | 0.08 | 0.06 | ||
| Mannitol | 6.43 | 6.20 | 96.64 | 0.01 | 1.24 | 6.20 | 96.69 | 0.03 | 1.39 | |
| 32.14 | 31.60 | 98.49 | 0.02 | 0.33 | 31.53 | 98.26 | 0.04 | 0.95 | ||
| 51.42 | 51.14 | 99.62 | 0.02 | 0.58 | 51.15 | 99.64 | 0.06 | 0.41 | ||
| Fructose | 3.26 | 2.504 | 76.81 | 0.01 | 4.64 | 2.50 | 76.63 | 0.04 | 18.04 | |
| 16.33 | 16.39 | 100.56 | 0.01 | 2.39 | 14.85 | 91.12 | 0.03 | 8.76 | ||
| 26.12 | 25.17 | 96.53 | 0.01 | 7.55 | 24.07 | 92.32 | 0.02 | 5.01 | ||
| Sorbose | 3.22 | 2.49 | 77.53 | 0.01 | 4.62 | 2.52 | 78.37 | 0.04 | 12.71 | |
| 16.09 | 16.29 | 101.42 | 0.01 | 1.56 | 15.51 | 96.57 | 0.03 | 4.91 | ||
| 25.74 | 24.73 | 96.25 | 0.01 | 5.43 | 23.98 | 93.33 | 0.02 | 4.65 | ||
| Analyte | Original (ng) | Spiked (ng) | Found a (ng) | Recovery b (%) | RSD(%) |
|---|---|---|---|---|---|
| Rhamnose | -c | 3.83 | 3.54 | 92.3 | 4.7 |
| Ribose | - | 3.89 | 3.61 | 93.0 | 3.9 |
| Arabinose | - | 3.87 | 3.67 | 94.7 | 5.6 |
| Xylose | - | 3.85 | 3.68 | 95.4 | 3.2 |
| Mannose | - | 3.89 | 4.06 | 104.5 | 3.4 |
| Glucose | 1.78 | 3.84 | 5.72 | 102.4 | 4.6 |
| Galactose | - | 3.90 | 3.76 | 96.3 | 6.1 |
| Mannitol | 34.85 | 7.71 | 41.97 | 92.2 | 4.6 |
| Fructose | - | 3.92 | 3.28 | 83.8 | 7.0 |
| Sorbose | - | 3.86 | 3.43 | 88.9 | 9.3 |
2.5. Compositional Sugars in Polysaccharides
| Samples | Ribose | Arabinose | Xylose | Mannose | Glucose | Galactose | |
|---|---|---|---|---|---|---|---|
| Natural C. sinensis | |||||||
| Zhongqiao | 1.00 | 16.61 | 1.28 | ||||
| Sichuan | 1.00 | 3.82 | 1.40 | ||||
| Qinghai | 1.00 | 4.33 | 1.31 | ||||
| Tibet | 1.00 | 13.65 | 1.60 | ||||
| Cultured C. sinensis | |||||||
| Wanfeng | 0.11 | 0.13 | 1.00 | 1.97 | 1.36 | ||
| Anhui | 0.02 | 0.14 | 0.07 | 1.00 | 1.09 | 1.11 | |
| Hebei | 0.24 | 0.11 | 1.00 | 2.60 | 3.03 | ||
| HongKong | 1.00 | 25.40 | 0.46 | ||||
| Huadong | 0.05 | 1.00 | 3.01 | 1.05 | |||
| Jiangxi | 0.32 | 0.15 | 1.00 | 1.18 | 1.96 | ||
| Cultured C. militaris | |||||||
| Aoli | 1.00 | 2.86 | 0.78 | ||||
| Xiankang | 0.01 | 1.00 | 2.17 | 0.93 | |||
| Quanxin | 0.14 | 1.00 | 1.28 | 1.07 | |||

2.6. Quantitation of Free Carbohydrates
| Analyte | Natural C. sinensis | Cultured C. sinensis | Cultured C. militaris | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zhongqiao | Sichuan | Qinghai | Tibet | Wanfeng | Anhui | Hebei | HongKong | Huadong | Jiangxi | Aoli | Xiankang | Quanxin | ||||
| Rhamnose | Fa | -b | - | - | - | - | - | - | - | - | - | - | - | - | ||
| C | - | - | - | - | - | - | - | - | - | - | - | - | - | |||
| Ribose | F | - | - | - | - | - | - | - | - | - | - | - | - | - | ||
| C | - | - | - | - | - | ± | 0.02 | - | ± | - | - | ± | ± | |||
| Arabinose | F | - | - | - | - | - | - | - | - | - | - | - | - | - | ||
| C | - | - | - | - | ± | ± | - | - | - | 0.23 | - | - | - | |||
| Xylose | F | - | - | - | - | - | - | - | - | - | - | - | - | - | ||
| C | - | - | - | - | ± | ± | ± | - | - | ± | - | - | - | |||
| Mannose | F | - | - | - | - | - | - | - | - | - | - | - | - | - | ||
| C | 0.15c | 0.19 | 0.24 | 0.25 | 0.60 | 0.72 | 0.42 | 0.20 | 0.46 | 0.89 | 0.24 | 0.28 | 0.32 | |||
| Glucose | F | 0.29 | ±d | ± | 0.13 | - | - | - | ± | - | - | ± | 0.13 | - | ||
| C | 2.51 | 0.73 | 1.03 | 3.37 | 1.17 | 0.79 | 1.09 | 5.03 | 1.39 | 1.05 | 0.70 | 0.60 | 0.41 | |||
| Galactose | F | - | - | - | - | - | - | - | - | - | - | - | - | - | ||
| C | 0.19 | 0.27 | 0.31 | 0.39 | 0.81 | 0.80 | 1.27 | ± | 0.48 | 1.74 | 0.19 | 0.26 | 0.34 | |||
| Mannitol | F | 8.14 | 9.76 | 7.99 | 10.74 | 5.83 | 4.01 | 4.44 | 0.94 | 2.24 | 4.30 | 3.16 | 4.09 | 3.96 | ||
| Fructose | F | - | - | - | - | - | - | - | - | - | - | - | - | - | ||
| C | - | - | - | - | - | - | - | - | - | - | - | - | - | |||
| Sorbose | F | - | - | - | - | - | - | - | - | - | - | - | - | - | ||
| C | - | - | - | - | - | - | - | - | - | - | - | - | - | |||
2.7. Quantitation of Conjunct Carbohydrates

2.8. Comparison of natural and cultured Cordyceps
3. Experimental Section
3.1. Materials and Chemicals
3.2. Pressurized Liquid Extraction
3.3. TFA Hydrolysis
3.4. Derivatization
3.5. GC-MS Analysis
3.6. Data Analysis
4. Conclusions
Acknowledgements
- Sample Availability: Samples of the compounds and materials are available from the authors.
References
- Cummings, J.H.; Stephen, A.M. Carbohydrate terminology and classification. Eur. J. Clin. Nutr. 2007, 61, S5–S18. [Google Scholar] [CrossRef]
- Wheeler, M.L.; Pi-Sunyer, F.X. Carbohydrate Issues: Type and Amount. J. Am. Diet. Assoc. 2008, 108, S34–S39. [Google Scholar]
- Kilcoyne, M.; Joshi, L. Carbohydrates in therapeutics. Cardiovasc. Hematol. Agents Med. Chem. 2007, 5, 186–197. [Google Scholar] [CrossRef]
- Zhang, M.; Cui, S.W.; Cheung, P.C.K.; Wang, Q. Antitumor polysaccharides from mushrooms a review on their isolation, structural characteristics and antitumor activity. Trends Food Sci. Tech. 2007, 18, 4–19. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Quinn, M.T. Botanical polysaccharides Macrophage immunomodulation and therapeutic potential. Int. Immunopharmacol. 2006, 6, 317–333. [Google Scholar] [CrossRef]
- Zhu, J.S.; Halpern, G.M.; Johns, K. The scientific rediscovery of an ancient Chinese herbal medicine: Cordyceps sinensis: Part I. J. Alt. Comp. Med. 1998, 4, 289–303. [Google Scholar] [CrossRef]
- Zhu, J.S.; Halpern, G.M.; Johns, K. The scientific rediscovery of an ancient Chinese herbal medicine: Cordyceps sinensis: Part II. J. Alt. Comp. Med. 1998, 4, 429–457. [Google Scholar] [CrossRef]
- Li, S.P.; Yang, F.Q.; Tsim, K.W.K. Quality control of Cordyceps sinensis, a valued traditional Chinese medicine. J. Pharm. Biomed. Anal. 2006, 41, 1571–1584. [Google Scholar] [CrossRef]
- Ng, T.B.; Wang, H.X. Pharmacological actions of Cordyceps, a prized folk. J. Pharm. Pharmacol. 2005, 57, 1509–1519. [Google Scholar]
- Paterson, R.R.M. Cordyceps – A traditional Chinese medicine and another fungal therapeutic biofactory? Phytochemistry 2008, 69, 1469–1495. [Google Scholar] [CrossRef] [Green Version]
- Kiho, T.; Ookubo, K.; Usui, S.; Ukai, S.; Hirano, K. Strcture features and hypoglycemic activity of a polysaccharide (CF-F10) from the cultured mycelium of Cordyceps sinensis. Biol. Pharm. Bull. 1999, 22, 966–970. [Google Scholar] [CrossRef]
- Kiho, T.; Ji, H.; Yamane, A.; Ukai, S. Polysaccharides in fungi. XXXII. Hypoglycemic activity and chemical properties of a polysaccharide from the cultural mycelium of Cordyceps sinensis. Biol. Pharm. Bull. 1993, 16, 1291–1293. [Google Scholar] [CrossRef]
- Li, S.P.; Zhao, K.J.; Ji, Z.N.; Song, Z.H.; Dong, T.T.X.; Lo, C.K.; Cheung, J.K.H.; Zhu, S.Q.; Tsim, K.W.K. A polysaccharide isolated from Cordyceps sinensis, a traditional Chinese medicine, protects PC12 cells against hydrogen peroxide-induced injury. Life Sci. 2003, 73, 2503–2513. [Google Scholar] [CrossRef]
- Li, S.P.; Zhang, G.H.; Zeng, Q.; Huang, Z.G.; Wang, Y.T.; Dong, T.T.X.; Tsim, K.W.K. Hypoglycemic activity of polysaccharide, with antioxidation, isolated from cultured Cordyceps mycelia. Phytomedicine 2006, 13, 428–433. [Google Scholar] [CrossRef]
- Yu, R.M.; Song, L.Y.; Zhao, Y.; Bin, W.; Wang, L.; Zhang, H.; Wu, Y.H.; Ye, W.C.; Yao, X.S. Isolation and biological properties of polysaccharide CPS-1 from cultured Cordyceps militaris. Fitoterapia 2004, 75, 465–472. [Google Scholar] [CrossRef]
- Chang, H.L.; Chao, G.R.; Chen, C.C.; Mau, J.L. Non-volatile components of Agaricus blazei, Antrodia camphorate and Cordyceps militaris mycelia. Food Chem. 2001, 74, 203–207. [Google Scholar] [CrossRef]
- Huang, S.J.; Tsai, S.Y.; Lee, Y.L.; Mau, J.L. Nonvolatile taste components of fruit bodies and mycelia of Cordyceps militaris. LWT-Food Sci. Tech. 2006, 39, 577–583. [Google Scholar] [CrossRef]
- Liu, G.P. Advance on the methods for determination of polysaccharides from Cordyceps. Yao Xue Shi Jian Za Zhi 2008, 26, 53–68. [Google Scholar]
- Fox, A. Carbohydrate profiling of bacteria by gas chromatography–mass spectrometry and their trace detection in complex matrices by gas chromatography-tandem mass spectrometry. J. Chromatogr. A 1999, 843, 287–300. [Google Scholar]
- Kim, J.S.; Laskowich, E.R.; Arumugham, R.G.; Kaiser, R.E.; MacMichael, G.J. Determination of saccharide content in pneumococcal polysaccharides and conjugate vaccines by GC-MSD. Anal. Biochem. 2005, 347, 262–274. [Google Scholar]
- Medeiros, P.M.; Simoneit, B.R.T. Analysis of sugars in environmental samples by gas chromatography–mass spectrometry. J. Chromatogr. A 1141, 271–278. [Google Scholar]
- Wang, Q.J.; Fang, Y.Z. Analysis of sugars in traditional Chinese drugs. J. Chromatogr. B 2004, 812, 309–324. [Google Scholar]
- Zhang, W.; He, H.; Zhang, X. Determination of neutral sugars in soil by capillary gas chromatography after derivatization to aldononitrile acetates. Soil Biol. Biochem. 2007, 39, 2665–2669. [Google Scholar] [CrossRef]
- Guerrant, G.O.; Moss, C.W. Determination of monosaccharides as aldononitrile, O-methyloxime, alditol, and cyclitol acetate derivatives by gas chromatography. Anal. Chem. 1984, 56, 636–638. [Google Scholar] [CrossRef]
- Varma, R.; Varma, R.S.; Wardi, A.H. Separation of aldononitrile acetates of neutral sugars by gas-liquid chromatography and its applications to polysaccharides. J. Chromatogr. 1973, 77, 222–227. [Google Scholar] [CrossRef]
- Seymour, F.R.; Chen, E.C.M.; Bishop, S.H. Identification of aldoses by use of their peracetylated aldononitrile derivatives: A G.L.C.-M.S. approach. Carbohydr. Res. 1979, 73, 19–45. [Google Scholar] [CrossRef]
- Seymour, F.R.; Chen, E.C.M.; Stouffer, J.E. Identification of ketoses by use of their peracetylated oxime derivatives: A G.L.C.-M.S. approach. Carbohydr. Res. 1980, 83, 201–242. [Google Scholar] [CrossRef]
- Seymour, F.R.; Unruh, S.L.; Nehlich, D.A. Quantitation of free sugars in plant tissue by G.L.C. of their peracetylated aldononitrile and ketoxime derivatives. Carbohydr. Res. 1989, 191, 175–189. [Google Scholar] [CrossRef]
- Li, T.L.; Wu, C.X.; Zhang, Y.X. Studies of gas chromatographic analysis of saccharides and alditols II. Some improvements in the analysis of acetylated aldononitriles by gas chromatography. Chin. J. Anal. Chem. 1981, 10, 272–276. [Google Scholar]
- Sanz, M.L.; Martínez-Castro, I. Recent developments in sample preparation for chromatographic analysis of carbohydrates. J. Chromatogr. A 2007, 1153, 74–89. [Google Scholar] [CrossRef]
- Ruiz-Matute, A.I.; Ramos, L.; Martínez-Castro, I.; Sanz, M.L. Fractionation of honey carbohydrates using pressurized liquid extraction with activated charcoal. J. Agric. Food Chem. 2008, 56, 8309–8313. [Google Scholar] [CrossRef]
© 2010 by the authors;
Share and Cite
Guan, J.; Yang, F.-Q.; Li, S.-P. Evaluation of Carbohydrates in Natural and Cultured Cordyceps by Pressurized Liquid Extraction and Gas Chromatography Coupled with Mass Spectrometry. Molecules 2010, 15, 4227-4241. https://doi.org/10.3390/molecules15064227
Guan J, Yang F-Q, Li S-P. Evaluation of Carbohydrates in Natural and Cultured Cordyceps by Pressurized Liquid Extraction and Gas Chromatography Coupled with Mass Spectrometry. Molecules. 2010; 15(6):4227-4241. https://doi.org/10.3390/molecules15064227
Chicago/Turabian StyleGuan, Jia, Feng-Qing Yang, and Shao-Ping Li. 2010. "Evaluation of Carbohydrates in Natural and Cultured Cordyceps by Pressurized Liquid Extraction and Gas Chromatography Coupled with Mass Spectrometry" Molecules 15, no. 6: 4227-4241. https://doi.org/10.3390/molecules15064227




