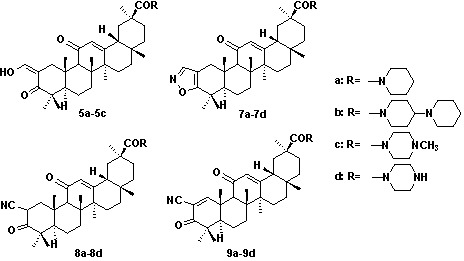
3. Experimental
3.1. General
GA (purity over 98%) was purchased from Shanghai Haokang Chemicals Co. Ltd., China. Other reagents were bought from commercial suppliers in analytic grade and used without further purification, unless noted otherwise. 1H-NMR and 13C-NMR spectra were recorded on a Bruker ARX-300 instrument with tetramethylsilane as an internal standard. Infra-red (IR) spectra were recorded on a Bruker IR-27G spectrometer. Mass spectra (MS) were determined on a Finnigan MAT/USA spectrometer (LC-MS). HRMS spectra were obtained on a Bruker micrOTOF-Q in an ESI mode. The melting points were determined on an electrically heated X4 digital visual melting point apparatus and are uncorrected. TLC plates (Alugram silica gel G/UV254) were purchased from Macherey-Nagel GmbH & Co.
3,11-Dioxoolean-12-en-30-oic acid (1). Jones’ reagent (15 mL) was added to a solution of GA (10.0 g, 21.2 mmol) in THF (35 mL). The solution was stirred at 0 ºC for 1 h and then was poured into H2O (100 mL). The precipitate was filtered and dried to afford compound 2 (9.5 g, 95.6% yield) as a white solid. 1H- NMR (CDCl3) δ (ppm): 5.76 (1H, s, H-12), , 2.97–2.94 (1H, m, H-1), 2.63–2.61 (1H, m, H-1), 2.45 (1H, s, H-9), 1.38, 1.27, 1.23, 1.17, 1.10, 1.07, 0.86 (s, CH3×7); LC-MS: 469.4 [M+H]+, 591.3 [M+Na]+.
Benzyl 3,11-dioxoolean-12-en-30-oate (2). BnBr (1.29 mL, 10.9 mmol) was added to a solution of 1 (5.0 g, 10.7 mmol) in DMF (50 mL). The mixture was stirred at 100 ºC for 3 h, cooled to room temperature and then poured into water (100 mL). The precipitate was filtered, washed with water to pH 7 and dried to give 2 (4.8 g, 80.9% yield) as a white solid. 1H-NMR (CDCl3) δ (ppm): 7.40–7.31 (5H, m, -CH2Ph), 5.58 (1H, s, H-12), 5.21 (1H, d, J = 12.3 Hz, -CH2Ph), 5.08 (1H, d, J = 12.3 Hz, -CH2Ph), 2.97–2.94 (1H, m, H-1), 2.42 (1H, s, H-9), 2.37–2.33 (1H, m, H-1), 1.40, 1.36, 1.27, 1.16, 1.15, 1.06, 0.75 (s, CH3×7); 13C-NMR (CDCl3): δ (ppm) 216.9 (C-3), 199.1 (C-11), 176.7 (C-30), 170. 2 (C-13), 136.0 (C-12), 128.5 (Ph), 128.3 (Ph), 128.2 (Ph), 128.1 (Ph), 101.1 (C-2); LC-MS: 559.4 [M+H]+, 581.4 [M+Na]+.
Benzyl 2-hydroxymethylene-3,11-dioxoolean-12-en-30-oate (3). NaH (3.84 g, 160 mmol) was added slowly to a solution of 2 (5.6 g, 10.1 mmol) in ethyl formate (100 mL). The mixture was stirred at r.t. for 10 min and methanol was added until no bubbles were generated. The mixture was diluted with a mixture of CH2Cl2 and Et2O (1:2) and washed three times with 5% aqueous HCl solution. The organic layer was evaporated in vacuum and purified on a silica gel column with cyclohexane-acetone (v/v) = 20:1 to give 3 (4.7 g, 80.0% yield) as a white solid. 1H-NMR (CDCl3) δ (ppm): 14.89 (s, 1H, OH), 8.61 (s, 1H, =CH-OH), 7.40-7.34 (m, 5H, -CH2Ph), 5.60 (s, 1H, H-12), 5.21 (d, 1H, J = 12.3 Hz, -CH2Ph), 5.07 (d, 1H, J = 12.3 Hz, -CH2Ph), 3.45 (d, 1H, J = 14.7 Hz, H-1), 2.44 (s, 1H, H-9), 1.35, 1.19, 1.16, 1.15, 1.13, 1.12, 0.75 (s, CH3×7); 13C-NMR (CDCl3): δ (ppm) 199.7 (C-11), 189.8 (C-3), 189.1 (C=CH-OH), 176.4 (C-30), 170.1 (C-13), 128.9 (Ph), 128.8 (Ph), 128.6 (Ph), 128.5 (-Ph), 128.3 (C-12), 106.1 (C-2), 66.4 (-CH2Ph), 59.8 (C-9); LC-MS: 585.3 [M-H]-.
2-Hydroxymethylene-3,11-dioxoolean-12-en-30-oic acid (4). 10% Pd/C (0.14 g) was added to a solution of 3 (0.76 g, 1.3 mmol) in THF (20 mL). The mixture was stirred at r.t. for 8 hr and filtered to remove Pd/C. The filtrate was concentrated in vacuum and the residue was purified on a silica gel column with petroleum ether : acetone (v/v) = 20:1 to give 4 (0.49 g, 76.3% yield) as a pink solid. 1H-NMR (CDCl3) δ (ppm): 14.89 (s, 1H, =CH-OH), 8.64 (s, 1H, =CH-OH), 5.79 (s, 1H, H-12), 3.47 (d, 1H, J = 15.0 Hz, H-1), 2.46 (s, 1H, H-9), 1.39, 1.25, 1.21, 1.19, 1.15, 1.14, 0.88 (s, CH3×7); 13C-NMR (CDCl3): δ (ppm) 200.0 (C-11), 189.8 (C-3), 189.2 (C=CH-OH), 182.7 (C-30), 170.3 (C-13), 128.8 (C-12), 106.1 (C-2), 59.9 (C-9); LC-MS: 495.4 [M-H]-.
3.2. General procedure for the preparation of 2-hydroxymethylene-30-amides 5a-5c
To a solution of 4 (2.34 g, 4 mmol) in chloroform (10 mL), oxalyl chloride (2 mL) was added. The mixture was stirred at r.t. for 1 hr and excess oxalyl chloride was removed by co-evaporation with hexane (three times). The obtained solid was dissolved in chloroform (30 mL) and an appropriate nitrogen heterocycle (6 mmol) was added. The mixture was stirred at r.t. for 5 min and washed three times with 5% aqueous HCl solution. The organic layer was dried over anhydrous MgSO4. After MgSO4 was removed by filtration, the filtrate was concentrated in vacuum and the residue was purified on a silica gel column with chloroform-methanol (v/v) = 50:1 to give a white solid. The Rf values were determined using TLC plates with chloroform-methanol (v/v) = 10:1.
N-(2-Hydroxymethylene-3,11-dioxoolean-12-en-30-yl)-piperidine (5a). Crystallization of the white solid from hexane and acetone (10:1) afforded white needles (1.6 g, 2.9 mmol). Yield: 72%; Rf = 0.4; m.p. 178–181 ºC; IR (KBr): 3425, 2927, 2867, 1612, 1446, 1417, 1382, 1362 cm-1; 1H-NMR (CDCl3): δ (ppm) 14.89 (d, 1H, J = 3.0 Hz, =CH-OH), 8.64 (d, 1H, J = 3.0 Hz, =CH-OH), 5.80 (s, 1H, H-12), 3.65-3.53 (m, 4H), 3.48 (d, 1H, J = 14.1 Hz, H-1), 2.45 (s, 1H, H-9), 1.38, 1.23, 1.21, 1.18, 1.15, 1.14, 0.85 (s, CH3×7); 13C-NMR (CDCl3): δ (ppm) 200.4 (C-11), 189.8 (C-3), 189.2 (CH=CH-OH), 174.0 (C-30), 171.4 (C-13), 129.8 (C-12), 106.1 (C-2), 59.9 (C-9); LC-MS: 562.6 [M-H]-; HRMS: m/z, calcd. for C36H52NO4 (M-H) 562.3890. Found: 562.3882.
N-(2-Hydroxymethylene-3,11-dioxoolean-12-en-30-yl)-4-piperidyl piperidine (5b). Crystallization of the white solid from hexane and acetone (10:1) afforded white needles (1.8 g, 2.8 mmol). Yield: 70%; Rf = 0.3; m.p. 210–212 ºC; IR (KBr): 3426.1, 2947.7, 2650.1, 2527.8, 1633.5, 1456.8 cm-1; 1H-NMR (CDCl3): δ (ppm) 14.89 (d, 1H, J = 3.0 Hz, =CH-OH), 8.63 (d, 1H, J = 3.0 Hz, =CH-OH), 5.73 (s, 1H), 4.54 (br, 2H), 3.45 (d, 1H, J = 14.7 Hz, H-1), 3.06-2.70 (m, 10H), 2.44 (s, 1H, H-9), 1.38, 1.23, 1.21, 1.17, 1.15, 1.13, 0.84 (s, CH3×7); LC-MS: 665.4 [M+NH4]+; HRMS: m/z, calcd. for C41H63N2O4 (M+H) 647.4782. Found: 647.4783.
N-(2-Hydroxymethylene-3,11-dioxoolean-12-en-30-yl)-4-methyl piperazine (5c). Crystallization of the white solid from hexane and acetone (5:1) afforded white needles (1.6 g, 2.7 mmol). Yield: 68%; Rf = 0.2; m.p. 155–157 ºC; IR (KBr): 3439, 2951, 2587, 1636, 1462, 1406, 1384 cm-1; 1H-NMR (CDCl3): δ (ppm) 14.90 (d, 1H, J = 3.0 Hz, =CH-OH), 8.64 (d, 1H, J = 3.0 Hz, =CH-OH), 5.80 (s, 1H), 3.67 (br, 4H), 3.49 (d, 1H, J = 14.7 Hz, H-1), 2.50–2.38 (m, 5H), 2.33 (s, 3H, N-CH3), 1.38, 1.23, 1.21, 1.18, 1.15, 1.14, 0.85 (s, CH3×7); LC-MS: 577.4 [M-H]-; HRMS: m/z, calcd. for C36H54N2O4 (M-H) 577.3999. Found: 577. 3990.
Isoxazolo[4, 5-b]olean-11-oxo 12-en-30-oic acid (6). A mixture of compound 4 (0.89 g, 0.9 mmol), NH2OH.HCl (0.36 g, 5.1 mol), and anhydrous sodium acetate (0.04 g, 0.49 mmol) were refluxed for 1.5 h in acetic acid (20 mL), cooled, and poured into ice-water. The precipitate was filtered, dried, and purified on a silica gel column with petroleum ether-acetone (v/v) = 20:1 to give 6 (0.74 g, 85.0% yield) as a white solid. 1H-NMR (CDCl3) δ (ppm): 12.07 (brs, 1H, -COOH), 8.03 (s, 1H, -CH=N-), 5.77 (s, 1H, H-12), 3.64 (d, 1H, J = 15.3 Hz, H-1), 2.56 (s, 1H, H-9), 1.41, 1.27, 1.25, 1.21, 1.18, 1.09, 0.86 (s, CH3×7); LC-MS: 492.4 [M-H]-.
3.3. General procedure for the preparation of isoxazolo[4,5-b]olean-11-oxo-12-en-30-amides 7a-7d
Oxalyl chloride (2 mL) was added to a solution of 6 (0.98 g, 2 mmol) in chloroform (10 mL). The mixture was stirred at r.t. for 1 hr and excess oxalyl chloride was removed by evaporation with hexane (three times). The obtained solid was dissolved in 30 mL chloroform and a nitrogen heterocycle (6 mmol) was added. The mixture was stirred at r.t. for 5 min and concentrated under vacuum. The residue was purified on a silica gel column with chloroform-methanol (v/v) = 50:1 to give a white solid. The Rf values were determined by TLC plates with chloroform-methanol (v/v) = 10:1.
N-(Isoxazolo[4,5-b]olean-11-oxo-12-en-30-yl)-piperidine (7a). Crystallization of the white solid from hexane afforded an amorphous solid (0.67 g, 60% yield). m.p. 148–151 ºC; Rf = 0.4; IR (KBr): 3429, 2929, 2853, 1628, 1462, 1411, 1383 cm-1; 1H-NMR (CDCl3): δ (ppm) 7.99 (s, 1H, -CH=N-), 5.81 (s, 1H, H-12), 3.65 (d, 1H, J = 15.7 Hz, H-1), 3.59 (br, 4H), 2.54 (s, 1H, H-9), 2.05 (m, 4H), 1.42, 1.38, 1.32, 1.24, 1.22, 1.18, 0.88 (s, CH3×7); 13C-NMR (CDCl3): δ (ppm) 199.3 (C-11), 173.6 (C-30), 172.2 (C-13), 170.4 (C-3), 150.3 (C-C=N), 128.6 (C-12), 109.1 (C-2); LC-MS: 559.4 [M-H]-; HRMS: m/z, calcd. for C36H51N2O3 (M-H) 559.3894. Found: 559.3903.
N-(Isoxazolo[4,5-b]olean-11-oxo-12-en-30-yl)-4-piperidyl piperidine (7b). Crystallization of the white solid from hexane afforded an amorphous solid (0.86 g, 67% yield). m.p. 158–162 ºC; Rf = 0.2; IR (KBr): 3439, 2932, 2853, 1724, 1630, 1456, 1413, 1383 cm-1; 1H-NMR (CDCl3): δ (ppm) 7.99 (s, 1H, -CH=N-), 5.79 (s, 1H, H-12), 4.51 (br, 2H), 3.65 (d, 1H, J = 15.7 Hz, H-1), 2.56 (s, 1H, H-9), 1.38, 1.32, 1.24, 1.22, 1.18, 1.10, 0.83 (s, CH3×6); 13C-NMR (CDCl3): δ (ppm) 200.7 (C-11), 173.6 (C-30),172.2 (C-13), 170.3 (C-3), 150.4 (C-C=N), 128.6 (C-12), 109.1 (C-2); LC-MS: 644.4 [M+H]+; HRMS: m/z, calcd. for C41H62N3O3 (M+H) 644.4785. Found: 643.4717.
N-(Isoxazolo[4,5-b]olean-11-oxo -12-en-30-yl)-4-methyl piperazine (7c). Crystallization of the white solid from hexane afforded an amorphous solid (0.58 g, 51% yield). m.p. 157–159 ºC; Rf = 0.2; IR (KBr): 3436, 2939, 2790, 1722, 1633, 1460, 1410.8, 1383.5 cm-1; 1H-NMR (CDCl3): δ (ppm) 7.99 (1H, s, -CH=N-), 5.81(s, 1H, H-12), 3.74 (br, 4H), 3.65 (d, 1H, J = 15.7 Hz, H-1), 2.53 (s, 3H, N-CH3), 2.41 (br, 4H), 1.38, 1.32, 1.24, 1.22, 1.18, 1.10, 0.85 (s, CH3×7); LC-MS: 576.5 [M+H]+, 598.5 [M+Na]+; HRMS: m/z, calcd. for C36H54N3O3 (M+H) 576.4159. Found: 576.4153.
N-(Isoxazolo[4,5-b]olean-11-oxo-12-en-30-yl)-piperazine (7d). Crystallization of the white solid from hexane afforded an amorphous solid (0.26 g, 50% yield). m.p. 216–218 ºC; Rf = 0.1; IR (KBr): 3427, 2968, 2866, 2467, 1725, 1637, 1459, 1409 cm-1; 1H-NMR (CDCl3): δ (ppm) 7.99 (1H, s, -CH=N-), 5.80 (s, 1H, H-12), 3.68 (m, 4H), 3.65 (d, 1H, J = 15.3 Hz, H-1), 2.91 (m, 4H), 2.54 (s, 1H, H-9), 1.38, 1.32, 1.24, 1.22, 1.18, 1.10, 0.85 (s, CH3×7); LC-MS: 562.3 [M+H]+; HRMS: m/z, calcd. for C35H52N3O3 (M+H) 562.4003. Found: 562.3999.
3.4. General procedure for the preparation of 2-cyano-3,11-dioxoolean-12-en-30-amides 8a-8d
NaOCH3 (7.25 g, 134 mmol) was added to a solution of isoxazolo[4,5-b]-olean-11-oxo-12-en- 30-amide (3.9 mmol) in MeOH (60 mL) and Et2O (125 mL). The mixture was stirred at r.t. for 8 hr and was extracted with a mixture of CH2Cl2 and Et2O (1:2). The extract was washed three times with 5% aqueous HCl solution and the acidic washings were re-extracted with a mixture of CH2Cl2 and Et2O (1:2). The combined organic layers were evaporated in vacuum and the precipitate was purified on a silica gel column with chloroform-methanol (v/v) = 50:1 to give a white solid. The Rf values were determined by TLC plates with chloroform-methanol (v/v) = 10:1.
N-(2-Cyano-3,11-dioxoolean-12-en-30-yl)-piperridine (8a). Crystallization of the white solid from hexane and EtOAc (10:1) afforded an amorphous solid (1.81 g, 83% yield). m.p. 163–165 ºC; Rf = 0.4; IR (KBr): 3434, 2931, 2855, 2359, 2204, 1722, 1629 cm-1; 1H-NMR (CDCl3): δ (ppm) 5.77 (s, 1H, H-12), 3.89 (m, 1H, H-2), 3.57 (br, 4H), 3.32 (m, 1H, H-1), 2.37 (s, 1H, H-9), 2.08 (m, 6H), 1.39, 1.35, 1.34, 1.22, 1.21, 1.10, 0.83 (s, CH3×7); 13C-NMR (CDCl3): δ (ppm) 207.4 (C-3), 197.8(C-11), 173.5(C-30), 171.3(C-13), 128.3 (C-12), 117.1(-CN), 79.6 (C-2), 61.3 (C-9); LC-MS: 559.4 [M-H]-; HRMS: m/z, calcd. for C36H53N2O3 (M-H) 559.3894. Found: 559.3898.
N-(2-Cyano-3,11-dioxoolean-12-en-30-yl)-4-piperidyl piperidine (8b). Crystallization of the white solid from hexane and EtOAc (10:1) afforded an amorphous solid (1.91 g, 76% yield). m.p. 189–191 ºC; Rf = 0.3; IR (KBr): 3423, 2948, 2647, 2528, 2202, 1627, 1457, 1417 cm-1; 1H-NMR (CDCl3): δ (ppm) 5.74 (s, 1H, H-12), 4.53 (br, 2H), 3.96 (m, 1H, H-2), 3.55 (m, 1H, H-1), 2.88-2.76 (br, 8H), 2.44-2.38 (m,4H), 2.36 (s, 1H, H-9),1.37, 1.35, 1.22, 1.18, 1.16, 1.11, 0.82 (s, CH3×7); 13C-NMR (CDCl3): δ (ppm) 205.2 (C-3), 198.6 (C-11), 173.8 (C-30), 170.8 (C-13), 128.1 (C-12), 117.1 (-CN), 79.5 (C-2), 63.1 (C-9); LC-MS: 644.7 [M+H]+; HRMS: m/z, calcd. for C41H62N3O3 (M+H) 644.4785. Found: 644.4789.
N-(2-Cyano-3,11-dioxoolean-12-en-30-yl)-4-methyl piperazine (8c). Crystallization of the white solid from hexane and EtOAc (10:1) afforded an amorphous solid (1.50 g, 67% yield). m.p. 197–199 ºC; Rf = 0.2; IR (KBr): 3425, 2947, 2792, 2202, 1719, 1633, 1460, 1410, 1383 cm-1; 1H-NMR (CDCl3): δ (ppm) 5.78 (s, 1H, H-12), 3.97 (m, 1H, H-2), 3.80 (br, 4H), 3.59 (m, 1H, H-1), 2.55 (s, 3H, N-CH3), 2.54 (s, 1H, H-9), 1.43, 1.38, 1.35, 1.18, 1.17, 1.15, 0.83 (s, CH3×7); 13C-NMR (CDCl3): δ (ppm) 205.2 (C-3), 198.6 (C-11), 173.9 (C-30), 170.7 (C-13), 128.2 (C-12), 117.1(-CN), 60.6 (C-9); LC-MS: 576.4 [M+H]+; HRMS: m/z, calcd. for C36H54N3O3 (M+H) 576.4159. Found: 576.4160.
N-(2-cyano-3,11-dioxoolean-12-en-30-yl)-piperazine (8d). Crystallization of the white solid from hexane and EtOAc (10:1) afforded an amorphous solid (0.88 g, 60% yield). m.p. 260–263 ºC; Rf = 0.1; IR (KBr): 3423, 2950, 2466, 2203, 1718, 1633, 1459 cm-1; 1H-NMR (CDCl3): δ (ppm) 5.77 (s, 1H, H-12), 3.97 (m, 1H, H-2), 3.71 (br, 4H), 3.62 (m, 1H, H-1), 2.95 (br, 4H), 2.41 (s, 1H, H-9), 1.43, 1.38, 1.35, 1.23, 1.18, 1.09, 0.83 (s, CH3×7); LC-MS: 562.6 [M+H]+; HRMS: m/z, calcd. for C35H52N3O3 (M+H) 562.4003. Found: 562.4009.
3.5. General procedure for the preparation of 2-cyano-3,11-dioxoolean-1,12-dien-30-amide derivatives 9a-9d
A mixture of 2-cyano-3,11-dioxoolean-12-en-30-amide derivative (3 mmol) and DDQ (98%) (0.77 g, 3.32 mmol) in dry toluene (80 mL) was heated under 80 ºC for 30 min. After insoluble material was removed by filtration, the filtrate was evaporated under vacuum to give a brown solid. The solid was purified on a silica gel column with chloroform-methanol (v/v) = 50:1. The Rf values were determined by TLC plates with chloroform-methanol (v/v) = 10:1.
N-(2-Cyano-3,11-dioxoolean-1,12-dien-30-yl)-piperidine (9a). Crystallization of the white solid from hexane and EtOAc (10:1) afforded an amorphous solid (0.94 g, 56% yield). m.p. 187–190 ºC; Rf = 0.4; IR (KBr): 3428, 2933, 2856, 2233, 1687, 1626, 1462 1412 cm-1; 1H-NMR (CDCl3): δ (ppm) 8.41 (s, 1H, H-1), 5.74 (s, 1H, H-12), 3.57 (br, 4H), 2.61 (s, 1H, H-9), 1.51, 1.37, 1.29, 1.21, 1.20, 1.15, 0.85 (s, CH3×7); 13C-NMR (CDCl3): δ (ppm) 197.6 (C-3), 197.5 (C-11), 176.1 (C-30), 172.5 (C-13), 168.5 (C-1), 123.4 (C-12), 114.8 (-CN), 113.3 (C-2), 53.4 (C9); LC-MS: 557.4 [M-H]-, 593.5 [M+Cl]-; HRMS: m/z, calcd. for C36H50N2O3 (M-H) 557.3737. Found: 5578.3733.
N-(2-Cyano-3,11-dioxoolean-1,12-dien-30-yl)-4-piperidyl piperidine (9b). Crystallization of the white solid from hexane and EtOAc (10:1) afforded an amorphous solid (0.98 g, 51% yield). m.p. 218–220 ºC; Rf = 0.3; IR (KBr): 3431, 2949, 2524, 2233, 1684, 1627, 1416 cm-1; 1H-NMR (CDCl3): δ (ppm) 8.40 (s, 1H, H-1), 5.73 (s, 1H, H-12), 4.58 (br, 2H), 3.14 (m, 1H), 2.85-2.78 (m, 4H), 2.61 (s, 1H, H-9), 2.27 (br, 4H), 1.51, 1.37, 1.31, 1.20, 1.19, 1.15, 0.83 (s, CH3×7); 13C-NMR (CDCl3): δ (ppm) 197.6 (C-3), 197.5 (C-11), 176.4 (C-30), 172.4 (C-13), 168.2 (C-1), 123.5 (C-12), 114.9 (-CN), 113.3(C-2); LC-MS: 642.6 [M+H]+; HRMS: m/z, calcd. for C41H60N3O3 (M+H+) 642.4629. Found: 642.4626.
N-(2-Cyano-3,11-dioxoolean-1,12-dien-30-yl)-4-methyl piperazine (9c). Crystallization of the white solid from hexane and EtOAc (10:1) afforded an amorphous solid (0.74 g, 43% yield). m.p. 185–187 ºC; Rf = 0.3; IR (KBr): 3438, 2950, 2680, 1719, 1685, 1633, 1461, 1408, 1384 cm-1; 1H-NMR (CDCl3): δ (ppm) 8.40 (s, 1H, H-1), 5.73 (s, 1H, H-12), 3.81 (br, 4H), 2.64 (br, 4H), 2.47 (s, 3H, -NCH3), 1.51, 1.38, 1.30, 1.21, 1.20, 1.15, 0.74 (s, CH3×7); LC-MS: 574.5[M+H]+; HRMS: m/z, calcd. for C36H52N3O3 (M+H) 574.4003. Found: 574.3997.
N-(2-Cyano-3,11-dioxoolean-1,12-dien-30-yl)-piperazine (9d). Crystallization of the white solid from hexane and EtOAc (10:1) afforded an amorphous solid (0.59 g, 40% yield). m.p. 201–203 ºC; Rf = 0.1; IR (KBr): 3426, 2951, 2468, 2233, 1721, 1686, 1638, 1459, 1384 cm-1; 1H-NMR (CDCl3): δ (ppm) 8.39 (s, 1H, H-1), 5.72 (s, 1H, H-12), 3.65 (br, 4H), 2.91 (br, 4H), 2.61 (s, 1H, H-9), 1.51, 1.49, 1.37, 1.30, 1.21, 1.20, 1.15, 0.74 (s, CH3×7); 13C-NMR (CDCl3): δ (ppm) 197.3 (C-3), 197.2 (C-11), 176.1 (C-30), 172.1 (C-13), 167.9 (C-1), 123.2 (C-12), 114.6 (-CN), 113.1 (C-2); LC-MS: 560.5 [M+H]+; HRMS: m/z, calcd. for C35H50N3O3 (M+H) 560.3846. Found: 560.3853.
3.6. Cell culture
HL-60 cells were cultured in RPMI 1640. The media were supplemented with 100 units/mL penicillin, 100 μg/mL streptomycin, 1 mmol/L L-glutamine, and 10% (v/v) heat-inactivated fetal bovine serum.
3.7. Cell growth inhibition assay
All compounds were dissolved in DMSO. A stock solution of 20 mmol/L of each compound was prepared in DMSO and stored in aliquots at -20 ºC. A working solution was diluted with ethanol and fresh medium before assaying. The final concentration of ethanol in the medium was less than 1% and the final concentration of DMSO was less than 0.1%. Cells were seeded at a density of 4 × 104 cells/mL in 24 well plates with various concentrations of the tested compounds and incubated for 3 days. Total cell number in each group was determined using a hemocytometer. The cell growth inhibitory ability was expressed as the ratio of the cell number in the groups treated with the compounds to that of cells treated with DMSO and/or ethanol. The concentration (GI50) which inhibited half of cell growth was calculated.