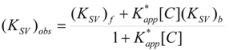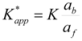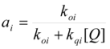Microheterogeneous Catalysis
Abstract
:1. Introduction

2. A Common Formulation for Homogeneous, Heterogeneous Catalysis and Electrocatalysis









 being the chemical potential of free R and
being the chemical potential of free R and  the chemical potential of R bound to the catalyst (see Equation 1). θ represents the association degree of R to C.
the chemical potential of R bound to the catalyst (see Equation 1). θ represents the association degree of R to C.



 and thus:
and thus:





 (see reference 29), consequently Equations 3 and 14 are the same. In the case of homogeneous or microheterogeneous catalysis, the rate of reaction according to previous equations would be given by:
(see reference 29), consequently Equations 3 and 14 are the same. In the case of homogeneous or microheterogeneous catalysis, the rate of reaction according to previous equations would be given by:



 , Equation 16 results in:
, Equation 16 results in:



 are the equilibrium constants corresponding to the adsorption of the reactant and transition state respectively, it is clear that:
are the equilibrium constants corresponding to the adsorption of the reactant and transition state respectively, it is clear that:



 is given by:
is given by:


























 , its chemical potential will be:
, its chemical potential will be:




 y
y  being the rate constants at the reference state, that is when φS = φM.
being the rate constants at the reference state, that is when φS = φM.






 and
and  :
:

 and
and  correspond to a reference state (the actual solution when ∆φ = 0) different from the customary one. These rate constants can be written as:
correspond to a reference state (the actual solution when ∆φ = 0) different from the customary one. These rate constants can be written as:


 and
and  , and the activity coefficients, referred to the customary reference state (of infinite dilution). In this way:
, and the activity coefficients, referred to the customary reference state (of infinite dilution). In this way:


 and αA and αD are the activities of the acceptor and donor, respectively.
and αA and αD are the activities of the acceptor and donor, respectively.
 .
.


3. Microheterogeneous Catalysis with Participation of Ground-State Reactants
3.1. General







 the rate constant for the unimolecular [40,41] process 60b. K1 and K2 represents the binding constants of the reactants to the micelles.
the rate constant for the unimolecular [40,41] process 60b. K1 and K2 represents the binding constants of the reactants to the micelles.
3.2. Micellar Solutions



















 ; (2) The ion-ion and ion head-group interaction are non-cooperative, in such a way that ion exchange rates are dependent only on the number of ions in a given aggregate and the concentrations of free ions in the bulk; (3) The degrees of ionization α of the individual micellar species MYiXi are the same independently of the i and j values. If m is the total number of occupied sites at the micellar surface:
; (2) The ion-ion and ion head-group interaction are non-cooperative, in such a way that ion exchange rates are dependent only on the number of ions in a given aggregate and the concentrations of free ions in the bulk; (3) The degrees of ionization α of the individual micellar species MYiXi are the same independently of the i and j values. If m is the total number of occupied sites at the micellar surface:
























































 (oi = oil/interface) and
(oi = oil/interface) and  (wi = water/interface) are defined as:
(wi = water/interface) are defined as:


 is high, in such a way that the ligand PADA can be considered distributed only between the oil and surfactant pseudophases. On the other hand, the Ni2+ and Co2+ ions are present only in the aqueous pseudophase and at the interface. This distribution is viewed as an ion exchange process between the counterions of the surfactant, Na+, and the M2+ cations, and quantitatively described by an equilibrium constant
is high, in such a way that the ligand PADA can be considered distributed only between the oil and surfactant pseudophases. On the other hand, the Ni2+ and Co2+ ions are present only in the aqueous pseudophase and at the interface. This distribution is viewed as an ion exchange process between the counterions of the surfactant, Na+, and the M2+ cations, and quantitatively described by an equilibrium constant  given by:
given by:



 vs. z should be linear. From the slope and intercept of this plot the authors were able to obtain K and
vs. z should be linear. From the slope and intercept of this plot the authors were able to obtain K and  . An interesting result is that K depends on w, that is, the structure of the interface depends on the water contents of the system [77].
. An interesting result is that K depends on w, that is, the structure of the interface depends on the water contents of the system [77].

 and
and  . These values, as K, depend on the water contents of the microemulsion. In fact
. These values, as K, depend on the water contents of the microemulsion. In fact  changes much more than
changes much more than  in such a way that variations of
in such a way that variations of  control the changes in the equilibrium constant.
control the changes in the equilibrium constant.3.3. Polymers and Related Microcatalysts


 (only the ruthenium complex binds to dendrimers) follow the expected trend according to the Pseudophase Model [44]. The same behavior is found when the oxidant is [Co(C2O4)3]3-, thus confirming that only the ruthenium complex binds to the dendrimers [89].
(only the ruthenium complex binds to dendrimers) follow the expected trend according to the Pseudophase Model [44]. The same behavior is found when the oxidant is [Co(C2O4)3]3-, thus confirming that only the ruthenium complex binds to the dendrimers [89].  has been studied in the presence of gold nanoparticles caped with N-(2-mercaptopropyonyl)glycine [60]. The kinetics, when the concentration of nanoparticles is changed, follows the trend corresponding to the equation of the Pseudophase Model with a high binding constant for the union of the ruthenium complex to the nanoparticles. According to the authors there is some anticooperative character in this union. However, this anticooperativity can be only formal in the sense that it could be due to saturation effects at the surface of the catalyst. The authors worked at different ionic strength in such a way that they were able to separate the electrostatic and non-electrostatic components of the binding free energy. According to their results, the electrostatic component is the main driving force for the union.
has been studied in the presence of gold nanoparticles caped with N-(2-mercaptopropyonyl)glycine [60]. The kinetics, when the concentration of nanoparticles is changed, follows the trend corresponding to the equation of the Pseudophase Model with a high binding constant for the union of the ruthenium complex to the nanoparticles. According to the authors there is some anticooperative character in this union. However, this anticooperativity can be only formal in the sense that it could be due to saturation effects at the surface of the catalyst. The authors worked at different ionic strength in such a way that they were able to separate the electrostatic and non-electrostatic components of the binding free energy. According to their results, the electrostatic component is the main driving force for the union. at pH = 5.4. In this case, it is the anion the ligand that binds to the nanoparticle. The rate of reaction changes by a factor of about ten in the presence of nanoparticles; this change decreasing as the ionic strength increases. Again, as in the case of gold nanoparticles, the main component of the free energy of binding is the electrostatic part.
at pH = 5.4. In this case, it is the anion the ligand that binds to the nanoparticle. The rate of reaction changes by a factor of about ten in the presence of nanoparticles; this change decreasing as the ionic strength increases. Again, as in the case of gold nanoparticles, the main component of the free energy of binding is the electrostatic part.3.4. Solutions Containing Saturable Receptors


 . In this case the complex [Ru(NH3)5Pz]2+ /calixarene has a 1:2 stoichiometry. When salt (NaCl) is added, a mixture of complexes with 1:1 and 1:2 stoichiometries is formed. Again, the Pseudophase Model can be used for rationalization of the results.
. In this case the complex [Ru(NH3)5Pz]2+ /calixarene has a 1:2 stoichiometry. When salt (NaCl) is added, a mixture of complexes with 1:1 and 1:2 stoichiometries is formed. Again, the Pseudophase Model can be used for rationalization of the results.3.5. Complex Systems







4. Microheterogeneous Catalysis with Participation of Excited States of Reactants
4.1. General














 depends on [Q] (through af and ab). Thus, unless koi>>kqi (i = f, b), curved Stern-Volmer plots may result in this case.
depends on [Q] (through af and ab). Thus, unless koi>>kqi (i = f, b), curved Stern-Volmer plots may result in this case.
4.2. Micellar Solutions







 , it follows that:
, it follows that:

















4.3. Polymer Solutions











4.4. Solutions Containing Saturable Receptors




4.5. Complex Systems





5. Conclusions
Acknowledgements
References
- Bunton, C.A.; Nome, F.; Quina, F.H.; Romsted, L.S. Ion binding and reactivity at charged aqueous interfaces. Acc. Chem. Res. 1991, 24, 357–364. [Google Scholar] [CrossRef]
- Marcus, R.A. Chemical and electrochemical electron-transfer theory. Annu. Rev. Phys. Chem. 1964, 15, 155–196. [Google Scholar] [CrossRef]
- Böttcher, C.J.F. Theory of Dielectric Polarization, 2nd ed; Elsevier: Amsterdam, Netherlands, 1973; Volume 1, Chapter 7; Volume 2, p. 318 and ff. [Google Scholar]
- Lao, K.Q.; Franzen, S.; Stanley, R.J.; Lambright, D.G.; Boxer, S.G. Effects of applied electric-fields on the quantum yields of the initial electron-transfer steps in bacterial photosynthesis . 1. Quantum yield failure. J. Phys. Chem. 1993, 97, 13165–13171. [Google Scholar] [CrossRef]
- Weaver, M.J. Dynamic solvent effects on activated electron-transfer reactions: principles, pitfalls, and progress. Chem. Rev. 1992, 92, 463–480. [Google Scholar] [CrossRef]
- Hazra, P.; Sarkar, N. Intramolecular charge transfer processes and solvation dynamics of Coumarin 490 in reverse micelles. Chem. Phys. Lett. 2001, 342, 303–311. [Google Scholar] [CrossRef]
- Biswas, R.; Rohman, N.; Pradhan, T.; Buchner, R. Intramolecular charge transfer reaction, polarity, and dielectric relaxation in AOT/water/heptane reverse micelles: pool size dependence. J. Phys. Chem. B 2008, 112, 9379–9388. [Google Scholar] [CrossRef]
- Bagchi, B.; Chandra, A. Collective orientational relaxation in dense dipolar liquids. Adv. Chem. Phys. 1991, 80, 1–126. [Google Scholar]
- Furse, K.E.; Corcelli, S.A. The dynamics of water at DNA interfaces: computational studies of Hoechst 33258 Bound to DNA. J. Am. Chem. Soc. 2008, 130, 13103–13109. [Google Scholar] [CrossRef]
- Moilanen, D.E.; Fenn, E.E.; Wong, D.; Fayer, M.D. Water dynamics at the interface in AOT reverse micelles. J. Phys. Chem. B 2009, 113, 8560–8568. [Google Scholar] [CrossRef]
- Sanchez, F.; Lopez-Lopez, M.; Perez-Tejeda, P. Effect of the micellar electric field on electron-transfer processes. a study of the metal-to-metal charge transfer within the binuclear complex pentaammineruthenium(III)-(mu-cyano)pentacyanoruthenium(II) in micellar solutions of sodium dodecylsulfate (SDS) and hexadecyltrimethylammonium chloride (CTACl). Langmuir 1998, 14, 3762–3766. [Google Scholar]
- Choudhury, S.D.; Kumbhakar, M.; Nath, S.; Sarkar, S.K.; Mukherjee, T.; Pal, H. Compartmentalization of reactants in different regions of sodium 1,4-bis(2-ethylhexyl)sulfosuccinate/heptane/water reverse micelles and its influence on bimolecular electron-transfer kinetics. J. Phys. Chem. B 2007, 111, 8842–8853. [Google Scholar]
- Marcus, R.A. Electron transfer reactions in chemistry. Theory and experiment. Pure and Applied Chem. 1997, 69, 13–29. [Google Scholar] [CrossRef]
- Muriel-Delgado, F.; Jimenez, R.; Gomez-Herrera, C.; Sanchez, F. Use of the Bronsted equation in the interpretation of micellar effects in kinetics (II). Study of the reaction [Fe(CN)5 4-CNpy] 3- + CN-reversible arrow Fe(CN)64- + 4-CNpy in CTACl micellar solutions. Langmuir 1999, 15, 4344–4350. [Google Scholar] [CrossRef]
- Grueso, E.; Prado-Gotor, R.; Lopez, M.; Gomez-Herrera, C.; Sanchez, F. DNA effects upon the reaction between acetonitrile pentacyanoferrate(II) and ruthenium pentammine pyrazine: Kinetic and thermodynamic evidence of the interaction of DNA with anionic species. Chem. Phys. 2005, 314, 101–107. [Google Scholar] [CrossRef]
- Bonan, C.; Germani, R.; Ponti, P.P.; Savelli, G.; Cerichelli, G.; Bacaloglu, R.; Bunton, C.A. Micellar headgroup size and anion nucleophilicity in SN2 reactions. J. Phys. Chem. 1990, 94, 5331–5336. [Google Scholar] [CrossRef]
- Mitra, R.K.; Sinha, S.S.; Verma, P.K.; Pal, S.K. Modulation of dynamics and reactivity of water in reverse micelles of mixed surfactants. J. Phys. Chem. B 2008, 112, 12946–12953. [Google Scholar] [CrossRef]
- Sinha, S.S.; Mitra, R.K.; Pal, S.K. Temperature-dependent simultaneous ligand binding in human serum albumin. J. Phys. Chem. B 2008, 112, 4884–4891. [Google Scholar] [CrossRef]
- Ramamurthy, V.; Eaton, D.F. Photochemistry and photophysics within cyclodextrin cavities. Acc. Chem. Res. 1988, 21, 300–306. [Google Scholar] [CrossRef]
- Zepik, H.H.; Walde, P.; Ishikawa, T. Vesicle formation from reactive surfactants. Angew. Chem. Int. Ed. 2008, 47, 1323–1325. [Google Scholar] [CrossRef]
- Hanczyc, M.M.; Fujikawa, S.M.; Szostak, J.W. Experimental models of primitive cellular compartments: encapsulation, growth, and division. Science 2003, 302, 618–622. [Google Scholar] [CrossRef]
- Carrasco, M.; Coca, R.; Cruz, I.; Daza, S.; Espina, M.; Garcia-Fernandez, E.; Guerra, F.J.; Leon, R.; Marchena, M.J.; Perez, I.; Puente, M.; Suarez, E.; Valencia, I.; Villalba, I.; Jimenez, R. Determination of the electrostatic potential difference between DNA and the solution containing it: a kinetic approach. Chem. Phys. Lett. 2007, 441, 148–151. [Google Scholar] [CrossRef]
- Lopez, P.; Sanchez, F.; Moya, M.L.; Jimenez, R. Common basis for salt, micelle and microemulsion effects upon the ionic reaction of hexachloroiridate(IV) with thiosulfate. J. Chem. Soc.Faraday Trans. 1996, 92, 3381–3384. [Google Scholar]
- Menger, F.M.; Portnoy, C.E. On chemistry of reactions proceeding inside molecular aggregates. J. Am. Chem. Soc. 1967, 89, 4698–4703. [Google Scholar] [CrossRef]
- Olson, A.R.; Simonson, T.R. Rates of ionic reactions in aqueous solutions. J. Chem. Phys. 1949, 17, 1167–1173. [Google Scholar] [CrossRef]
- Frost, A.A.; Pearson, R.G. Kinetics and Mechanism: A Study of Homogeneous Chemical Reactions; John Wiley & Sons: New York, NY, USA, 1996; p. 181, and ff. [Google Scholar]
- Scatchard, G. Equilibria and reaction rates in dilute electrolyte solutions. Natl. Bur. Standar U.S. Circ. 1953, 524, 185–192. [Google Scholar]
- Bronsted, J.N. On the theory of the chemical reaction rate. Z. Phys. Chem. 1922, 102, 169–207. [Google Scholar]
- Marchena, M.; Sanchez, F. The Bronsted equation: The universal equation? Prog. React. Kinet. Mech. 2006, 31, 221–248. [Google Scholar]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. Part I. solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Bockris, J.O’M.; Reddy, A.K.N. Modern Electrochemistry; Plenum Press: New York, NY, USA, 1970; Volume 2, p. 1141, and ff. [Google Scholar]
- Butler, J.A.V. Studies in heterogeneous equilibria. Part II: the kinetic interpretation of the Nernst theory of electromotive force. Trans. Faraday Soc. 1924, 19, 729–733. [Google Scholar] [CrossRef]
- Erdey-Gruz, T.; Volmer, M. The theory of hydrogen high tension. Z. Physik. Chem. 1930, 150, 203–213. [Google Scholar]
- Lopez-Lopez, M.; Lopez-Cornejo, P.; Garcia, A.; Sanchez, F. Determination of substrate/ligand binding constants from electromotrive force measurements. J. Sol. Chem. 2008, 37, 519–526. [Google Scholar] [CrossRef]
- Almgren, M.; Grieser, F.; Thomas, J.K. One-electron redox potentials and rate of electron-transfer in aqueous micellar solution - partially solubilized quinones. J. Phys. Chem. 1979, 83, 3232–3236. [Google Scholar] [CrossRef]
- Davies, K.; Hussam, A. Electrochemical studies of metal-complexes in sodium dodecyl-sulfate micellar solution. Langmuir 1993, 9, 3270–3276. [Google Scholar] [CrossRef]
- Carbone, A.I.; Cavasino, F.P.; Sbriziolo, C.; Pelizzetti, E. Equilibrium and kinetic-studies of the electron-transfer reactions involving ferrocene and cobalt(III) complexes in micellar solutions. J. Phys. Chem. 1985, 89, 3578–3582. [Google Scholar] [CrossRef]
- Herrera, C.G.; Jimenez, R.; Perez-Tejeda, P.; Lopez-Cornejo, P.; Prado-Gotor, R.; Sanchez, F. On the equivalence of the pseudophase related models and the Bronsted approach in the interpretation of reactivity under restricted geometry conditions. Prog. React. Kinet. Mech. 2004, 29, 289–310. [Google Scholar]
- Miller, D.D.; Evans, D.F. Fluorescence quenching in double-chained surfactants theory of quenching in micelles and vesicles. J. Phys. Chem. 1989, 93, 323–333. [Google Scholar] [CrossRef]
- Miller, D.D.; Magid, L.J.; Evans, D.F. Fluorescence quenching in double-chained surfactants 2. Experimental results. J. Phys. Chem. 1990, 94, 5921–5930. [Google Scholar]
- de la Vega, R.; Lopez-Cornejo, P.; Perez-Tejeda, P.; Sanchez, A.; Prado, R.; Lopez, M.; Sanchez, F. Influence of the micellar electric field on electron-transfer processes (ii): a study of the Ru(NH3)5Pz2++Co(C2O4)33- reaction in SDS micellar solution containing NaCl. Langmuir 2000, 16, 7986–7990. [Google Scholar] [CrossRef]
- Lopes-Costa, T.; Sanchez, F.; Lopez-Cornejo, P. Cooperative and noncooperative binding of *[Ru(Bpy)3]2+ to DNA and SB4.5G dendrimers. J. Phys. Chem. B 2009, 113, 9373–9378. [Google Scholar]
- Lopez-Comejo, P.; Perez, P.; Garcia, F.; de la Vega, R.; Sanchez, F. Use of the pseudophase model in the interpretation of reactivity under restricted geometry conditions. an application to the study of the [Ru(NH3)Pz]2++ S2O82- electron-transfer reaction in different microheterogeneous systems. J. Am. Chem. Soc. 2002, 124, 5154–5164. [Google Scholar] [CrossRef]
- Armitage, B.A. DNA Binders and Related Subjects; Waring, M.J., Chaires, J.B., Eds.; Springer Verlas: New York, Berlin, 2005; (Top. Curr. Chem. 2005, 263, 55). [Google Scholar]
- Lavery, R. Multiple Aspects of DNA and RNA: from Biophysics to Bioinformatics; Chatenay, D., Cocco, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Garcia-Rio, L.; Mejuto, J.C.; Perez-Lorenzo, M. Simultaneous effect of microemulsions and phase-transfer agents on aminolysis reactions. J. Phys. Chem. B 2007, 111, 11149–11156. [Google Scholar] [CrossRef]
- Graciani, M.D.; Rodriguez, M.A.; Moya, M.L. Study of the ligand substitution reaction Fe(CN)5H2O3- plus pyrazine in micellar solutions. Int. J. Chem. Kinet. 1997, 29, 377–384. [Google Scholar] [CrossRef]
- Prado-Gotor, R.; Jimenez, R.; Perez-Tejeda, P.; Lopez-Cornejo, P.; Lopez-Lopez, M.; Sanchez, A.; Muriel-Delgado, F.; Sanchez, F. Electron transfer reactions in micellar systems. Prog. React. Kinet. Mech. 2000, 25, 371–407. [Google Scholar]
- Barzykin, A.V.; Tachiya, M. Unified treatment of luminescence quenching kinetics in micelles with quencher migration on the basis of a generalized Smoluchowski approach. J. Phys. Chem. B 1998, 102, 1296–1300. [Google Scholar] [CrossRef]
- Alargova, R.G.; Petkov, J.T.; Petsev, D.N. Micellization and interfacial properties of alkyloxyethylene sulfate surfactants in the presence of multivalent counterions. J. Colloid Interface Sci. 2003, 261, 1–11. [Google Scholar] [CrossRef]
- Benjamin, I. Chemical reactions and solvation at liquid interfaces: A microscopic perspective. Chem. Rev. 1996, 96, 1449–1475. [Google Scholar] [CrossRef]
- Abuin, E.; Lissi, E.; Ceron, A.; Rubio, M.A. Spectroscopic probing of the effect of alkanols on the properties of the head group region in reverse micelles of AOT-heptane-water. J. Colloid Interface Sci. 2003, 258, 363–366. [Google Scholar] [CrossRef]
- Hartley, G.S.; Roe, J.W. Ionic concentrations at interfaces. Trans. Faraday Soc. 1940, 35, 101–109. [Google Scholar] [CrossRef]
- Quina, F.H.; Chaimovich, H. Ion-exchange in micellar solutions .1. Conceptual-framework for ion-exchange in micellar solutions. J. Phys. Chem. 1979, 83, 1844–1850. [Google Scholar] [CrossRef]
- Lopez-Cornejo, P.; Sanchez, F. Micellar effects on the kinetics of the oxidation of the excited state of the [Ru(Bpy)3]2+ complex by S2O82-. A comparison of different approaches for the interpretation of micellar effects on kinetics. J. Phys. Chem. B 2001, 105, 10523–10527. [Google Scholar] [CrossRef]
- Mayer, J.E. The theory of ionic solutions. J. Chem. Phys. 1950, 18, 1426–1436. [Google Scholar] [CrossRef]
- de la Vega, R.; Perez-Tejeda, P.; Lopez-Cornejo, P.; Sanchez, F. Kinetic study of the oxidation of [Ru(NH3)5Pz]2+ by [Co(C2O4)3]3- in AOT-oil-water microemulsions and in CTAC1 micellar solutions. Langmuir 2004, 20, 1558–1563. [Google Scholar]
- Howegrant, M.; Lippard, S.J. Binding of platinum(II) intercalation reagents to deoxyribonucleic-acid - dependence on base-pair composition, nature of the intercalator, and ionic-strength. Biochemistry 1979, 18, 5762–5769, (N. Y.). [Google Scholar] [CrossRef]
- Grueso, E.; Alcantara, D.; Martinez, J.; Mancera, M.; Penades, S.; Sanchez, F.; Pradogotor, R. Kinetic approach for the study of noncovalent interaction between [Ru(Nh3)5pz]2+ and gold nanoparticles. J. Phys. Chem. A 2007, 111, 9769–9774. [Google Scholar]
- Lopez-Cornejo, P.; Prado-Gotor, R.; Garcia-Santana, A.; Perez, F.; Sanchez, F. Comparative study of micellar and DNA effects on the reaction [Ru(NH3)5py]2++S2O82-. Langmuir 2003, 19, 3185–3189. [Google Scholar]
- Rodenas, E.; Ortega, F.; Vera, S.; Otero, C.; Maestro, S. Surfactants in solution; Mittal, K.L., Ed.; Plenum Pub. Co: New York, NY, USA, 1989; volume. 9, p. 211, and ff. [Google Scholar]
- Ortega, F.; Rodenas, E. An electrostatic approach for explaining the kinetic results in the reactive counterion surfactants CTAOH and CTACN. J. Phys. Chem. 1987, 91, 837–840. [Google Scholar] [CrossRef]
- Rodriguez, A.; del Mar Graciani, M.; Bitterman, K.; Carmona, A.T.; Moya, M.L. Micellar kinetic effects in gemini micellar solutions: influence of sphere-to-rod transitions on kinetics. J. Colloid Interface Sci. 2007, 313, 542–550. [Google Scholar] [CrossRef]
- Graciani, M.D.; Rodriguez, A.; Moya, M.L. Study of the reaction between methyl 4-nitrobenzenesulfonate and bromide ions in mixed single-chain-gemini micellar solutions: kinetic evidence for morphological transitions. J. Colloid Interface Sci. 2008, 328, 324–330. [Google Scholar] [CrossRef]
- Rodriguez, A.; Graciani, M.D.; Munoz, M.; Robina, I.; Moya, M.L. Effects of ethylene glycol addition on the aggregation and micellar growth of gemini surfactants. Langmuir 2006, 22, 9519–9525. [Google Scholar] [CrossRef]
- Lopez-Cornejo, P.; Prado-Gotor, R.; Gomez-Herrera, C.; Jimenez, R.; Sanchez, F. Influence of the charge and concentration of coreactants on the apparent binding constant of the reactant to micelles. Langmuir 2003, 19, 5991–5995. [Google Scholar] [CrossRef]
- Piszkiewicz, D. Micelle catalyzed reactions are models of enzyme catalyzed reactions which show positive homotropic interactions. J. Am. Chem. Soc. 1976, 98, 3053–3055. [Google Scholar] [CrossRef]
- Piszkiewicz, D. Positive cooperativity in micelle-catalyzed reactions. J. Am. Chem. Soc. 1977, 99, 1550–1557. [Google Scholar] [CrossRef]
- Piszkiewicz, D. Cooperativity in bimolecular micelle-catalyzed reactions: inhibition of catalysis by high-concentrations of detergent. J. Am. Chem. Soc. 1977, 99, 7695–7697. [Google Scholar] [CrossRef]
- Hill, A.V. The possible effects of the aggregation of molecules of hemoglobin on its dissociation curve. J. Physiol. (Lond) 1910, 40, 4. [Google Scholar]
- Lopez, P.; Sanchez, F.; Moya, M.L.; Jimenez, R. Common basis for salt, micelle and microemulsion effects upon the ionic reaction of hexachloroiridate(iv) with thiosulfate. J. Chem. Soc. Faraday Trans. 1996, 92, 3381–3384. [Google Scholar] [CrossRef]
- Lopez-Cornejo, P.; Mozo, J.D.; Roldan, E.; Dominguez, M.; Sanchez, F. Kinetic study of the reaction *[Ru(Bpy)3]2++S2O82- in solutions of brij-35 at premicellar and micellar concentrations. Chem. Phys. Lett. 2002, 352, 33–38. [Google Scholar] [CrossRef]
- Biresaw, G.; Bunton, C.A. Size vs reactivity in organized assemblies-deacylation and dephosphorylation in functionalized assemblies. J. Phys. Chem. 1986, 90, 5849–5853. [Google Scholar] [CrossRef]
- Valero, M.; Sanchez, F.; Gomez-Herrera, C.; Lopez-Cornejo, P. Study of water solubilized in AOT/n-decane/water microemulsions. Chem. Phys. 2008, 345, 65–72. [Google Scholar] [CrossRef]
- Marchena, M.; Sanchez, F. Kinetic effects of methyl-beta-cyclodextrin and 2-hydroxypropyl-beta-cyclodextrin and their mixtures on the reaction [Fe(CN)5(4-Phepy)]3- + [Co(NH3)4(H2O)2]3+. Chem. Phys. Lett. 2009, 471, 234–238. [Google Scholar] [CrossRef]
- Fernandez, E.; Garcia-Rio, L.; Knorl, A.; Leis, J.R. Metal-ligand complexation in water-in-oil microemulsions. i. thermodynamic approach. Langmuir 2003, 19, 6611–6619. [Google Scholar]
- Fernandez, E.; Garcia-Rio, L.; Knorl, A.; Leis, J.R. Kinetic study of ni2+ and co2+ complexation by pada in AOT-based water-in-oil microemulsions. Eur. J. Org. Chem. 2005, 4, 740–748. [Google Scholar]
- Secco, F.; Venturini, M.; Lopez, M.; Perez, P.; Prado, R.; Sanchez, F. Effect of DNA on the rate of electron transfer reactions between non-intercalated reactants: kinetic study of the reactions Reactions [Ru(NH3)5Pz]2++[Co(C2O4)3]3- and [Ru(NH3)5Py]2++[Co(NH3)4pzCO2]2+ in aqueous solutions in the presence of DNA. Phys. Chem. Chem. Phys. 2001, 3, 4412–4417. [Google Scholar]
- De la Vega, R.; Perez, P.; Prado-Gotor, R.; Sanchez, F. DNA interactions with small solutes: change in the character of the binding of [Ru(NH3)5Pz]2+ to DNA as a consequence of changes in the solvent. Chem. Phys. 2004, 297, 163–169. [Google Scholar] [CrossRef]
- Sun, Y.S.; Landry, J.P.; Fei, Y.Y.; Zhu, X.D. Effect of fluorescently labeling protein probes on kinetics of protein-ligand reactions. Langmuir 2008, 24, 13399–13405. [Google Scholar] [CrossRef]
- Grueso, E.; Sanchez, F.; Martin, V. I.; Garcia-Fernandez, E.; Prado-Gotor, R. Quantification of salts and cosolvents-DNA interactions in terms of free energies: a study using the pyren-1-carboxyaldehyde as fluorescent probe. Chem. Phys. 2008, 352, 306–310. [Google Scholar] [CrossRef]
- Tan, Z.J.; Chen, S.J. Nucleic acid helix stability: effects of salt concentration, cation valence and size, and chain length. Biophys. J. 2006, 90, 1175–1190. [Google Scholar] [CrossRef]
- Grueso, E.; Sanchez, F. DNA-surfactant interactions: a procedure for determination group contributions. J. Phys. Chem. B 2008, 112, 698–702. [Google Scholar] [CrossRef]
- Hays, M.E.; Jewell, C.M.; Lynn, D.M.; Abbott, N.L. Reversible condensation of DNA using a redox-active surfactant. Langmuir 2007, 23, 5609–5614. [Google Scholar] [CrossRef]
- Haynil, D.T. Biological thermodynamic; Cambridge University Press, 2004; p. 223, and ff. [Google Scholar]
- Ortiz, J.; Guichou, J.F.; Chavanieu, A.; Sanchez, F.; Prado-Gotor, R. Polymerization-induced enhancement of binding and binding-induced polymerization. Chem. Phys. Lett. 2004, 384, 266–270. [Google Scholar] [CrossRef]
- Chavanieu, A.; Guichou, J.F.; Prado-Gotor, R.; Perez-Tejeda, P.; Jimenez, R.; Lopez-Cornejo, P.; Sanchez, F. Strength and character of peptide/anion interactions. J. Phys. Chem. B 2005, 109, 19676–19680. [Google Scholar]
- de la Vega, R.; Perez-Tejeda, P.; Prado-Gotor, R.; Lopez-Cornejo, P.; Jimenez, R.; Perez, F.; Sanchez, F. Effects of SB1.5G and SB4.5G dendrimers on the rate of the electron transfer reaction between [Ru(NH3)5Pz]2+ and [Co(C2O4)3]3-. Chem. Phys. Lett. 2004, 398, 82–86. [Google Scholar] [CrossRef]
- Jimenez, R.; Garcia-Fernandez, E.; Sanchez, F. Dendrimer effects upon the reaction between (acetonitrile) pentacyano-ferrate(II) and pentaamminepyrazine-ruthenium(II). Chem. Phys. Lett. 2006, 420, 372–376. [Google Scholar] [CrossRef]
- Sanchez, R.; Villar, M.; Guiraum, A.; Prado-Gotor, R. Restricted geometry conditions promoted by alooh nanoparticles: variable strength and character of alooh-cluster/charged ligand interactions as a consequence of changes in the solvent. J. Phys. Chem. C 2008, 112, 9240–9246. [Google Scholar]
- Miyoshi, H.; Matsuo, Y.; Liu, Y.Y.; Sakata, T.; Mori, H. Behavior of fluorescent molecules bound to the interior of silica nanocapsules in various solvents. J. Colloid Interface Sci. 2009, 331, 507–513. [Google Scholar] [CrossRef]
- Martin, C.; Sanchez, F.; Jimenez, R.; Prado, R.; Perez-Tejeda, P.; Lopez-Cornejo, P. Salt and solvent effects on the kinetics and thermodynamics of the inclusion of the ruthenium complex [Ru(NH3)5(4,4 '-Bpy)]2+ in beta-cyclodextrin. J. Phys. Chem. B 2006, 110, 12959–12963. [Google Scholar]
- Connors, K.A. The stability of cyclodextrin complexes in solution. Chem. Rev. 1997, 97, 1325–1357. [Google Scholar] [CrossRef]
- de Namor, A.F.D.; Chahine, S.; Castellano, E.E.; Piro, O.E. Thermodynamics of host-guest interactions in lower rim functionalized calix[4]arenes and metal cations: the medium effect. J. Phys. Chem. B 2004, 108, 11384–11392. [Google Scholar]
- Dey, J.; Roberts, E.L.; Warner, I.M. Effect of sodium perchlorate on the binding of 2-(4'-aminophenyl)- and 2-(4'-(n,n'-dimethylamino)phenyl)benzothiazole with beta-cyclodextrin in aqueous solution. J. Phys. Chem. A 1998, 102, 301–305. [Google Scholar]
- Lima, S.; Goodfellow, B.J.; Teixeira-Dias, J.J.C. How inorganic anions affect the inclusion of hexanoic and decanoic acid in beta-cyclodextrin. J. Phys. Chem. A 2004, 108, 10044–10049. [Google Scholar] [CrossRef]
- Rekharsky, M.V.; Inoue, Y. Solvent and guest isotope effects on complexation thermodynamics of alpha-, beta-, and 6-amino-6-deoxy-beta-cyclodextrins. J. Am. Chem. Soc. 2002, 124, 12361–12371. [Google Scholar] [CrossRef]
- Breslow, R.; Dong, S.D. Biomimetic reactions catalyzed by cyclodextrins and their derivatives. Chem. Rev. 1998, 98, 1997–2011. [Google Scholar] [CrossRef]
- Takahashi, K. Organic reactions mediated by cyclodextrins. Chem. Rev. 1998, 98, 2013–2033. [Google Scholar] [CrossRef]
- Iglesias, E. Cyclodextrins as enzyme models in nitrosation and in acid-base-catalyzed reactions of alkyl nitrites. J. Am. Chem. Soc. 1998, 120, 13057–13069. [Google Scholar] [CrossRef]
- Ramachandran, M.S. Lathakannan Beta-cyclodextrin-catalyzed decomposition of caro's acid. Int. J. Chem. Kinet. 2002, 34, 508–513. [Google Scholar] [CrossRef]
- Sueishi, Y.; Hishikawa, H. Complexation of 4-dimethylaminoazobenzene with various kinds of cyclodextrins: effects of cyclodextrins on the thermal cis-to-trans isomerization. Int. J. Chem. Kinet. 2002, 34, 481–487. [Google Scholar] [CrossRef]
- Tee, O.S.; Gadosy, T.A. Acyl transfer mediated by complexation: The effect of cyclodextrins on the reaction of nucleophiles with p-nitrophenyl acetate and hexanoate. J.e Chem. Soc. Perkin Transactions 2 1994, 2307–2311. [Google Scholar]
- Tee, O.S. The stabilization of transition states by cyclodextrins and other catalysts. Adv.n Phys. Org. Chem. 1994, 29, 1–85. [Google Scholar] [CrossRef]
- Tee, O.S. The binding of transition-states by cyclomalto-oligosaccharides. Carbohydr. Res. 1989, 192, 181–195. [Google Scholar] [CrossRef]
- Imonigie, J.A.; Macartney, D.H. Effects of cyclodextrin inclusion on the kinetics of the outer-sphere oxidation of 4-tert-butylcatechol by transition-metal complexes in acidic aqueous-media. Inorg. Chem. 1993, 32, 1007–1012. [Google Scholar] [CrossRef]
- Kaifer, A.E. Interplay between molecular recognition and redox chemistry. Acc. Chem. Res. 1999, 32, 62–71. [Google Scholar] [CrossRef]
- Nielson, R.M.; Hupp, J.T.; Yoon, D.I. Modulation of outer-sphere electron-transfer reactivity via primitive molecular recognition effects:Ru(NH3)5(4-Methyl-Pyridine)3+/2+ self-exchange kinetics in the presence of macrocyclic polyether species. J. Am. Chem. Soc. 1995, 117, 9085–9086. [Google Scholar] [CrossRef]
- Shortreed, M.E.; Wylie, R.S.; Macartney, D.H. Inclusion of (N-adamantan-1'-ylpyrazinium)pentacyanoferrate(II) ion in alpha-cyclodextrins and beta-cyclodextrins. Effects of inclusion on the spectroscopic properties and ligand substitution kinetics. Inorg. Chem. 1993, 32, 1824–1829. [Google Scholar] [CrossRef]
- Lonetti, B.; Lo Nostro, P.; Ninham, B.W.; Baglioni, P. Anion effects on calixarene monolayers: a hofmeister series study. Langmuir 2005, 21, 2242–2249. [Google Scholar] [CrossRef]
- Izatt, R.M.; Pawlak, K.; Bradshaw, J.S.; Bruening, R.L. Thermodynamic and kinetic data for macrocycle interaction with cations and anions. Chem. Rev. 1991, 91, 1721–2085. [Google Scholar]
- Casnati, A.; Sansone, F.; Ungaro, R. Peptido- and glycocalixarenes: Playing with hydrogen bonds around hydrophobic cavities. Acc. Chem. Res. 2003, 36, 246–254. [Google Scholar] [CrossRef]
- Sanchez, A.; Jimenez, R.; Ternero, F.; Mesa, R.; Pinero, C. A.; Muriel, F.; Lopez-Cornejo, P. Rigidity and/or flexibility of calixarenes. effect of the p-sulfonatocalix[n]arenes (n = 4, 6, and 8) on the electron transfer process [Ru(NH3)5Pz]2++[Co(C2O4)3]3-. J. Phys. Chem. B 2007, 111, 10697–10702. [Google Scholar]
- Lopez-Cornejo, P.; Bote, B.; Felix, R.; Infantes, I.; Lopez, P.; Martin, A.; Mateos, E.; Perez, M.; Rojas, A.; Suarez, R. Binding of [Ru(NH3)5Pz]2+to 4-sulfocalix[4]arene sodium salt. effects of the host-guest interaction on electron transfer processes. J. Phys. Chem. B 2009, 113, 12721–12726. [Google Scholar]
- Rodriguez, A.; Munoz, M.; Graciani, M.D.M.; Moya, M.L. Kinetic micellar effects in tetradecyltrimethylammonium bromide-pentanol micellar solutions. J. Colloid Interface Sci. 2002, 248, 455–461. [Google Scholar] [CrossRef]
- Munoz, M.; Rodriguez, A.; Graciani, M.D.; Moya, M.L. Micellar medium effects on the hydrolysis of phenyl chloroformate in ionic, zwitterionic, nonionic, and mixed micellar solutions. Int. J. Chem. Kinet. 2002, 34, 445–451. [Google Scholar] [CrossRef]
- Munoz, M.; Graciani, M.D.; Rodriguez, A.; Moya, M.L. Influence of the addition of alcohol on the reaction methyl-4-nitrobenzenesulfonate plus Br- in tetradecyltrimethylammonium bromide aqueous micellar solutions. J. Colloid Interface Sci. 2003, 266, 208–214. [Google Scholar] [CrossRef]
- Fernandez, G.; Rodriguez, A.; Graciani, M.D.; Munoz, M.; Moya, M.L. Study of the reaction methyl 4-nitrobenzene-sulfonate plus cl- in mixed hexadecyltrimethyl-ammonium chloride-triton x-100 micellar solutions. Int. J. Chem. Kinet. 2003, 35, 45–51. [Google Scholar] [CrossRef]
- Munoz, M.; Rodriguez, A.; Graciani, M.D.; Moya, M.L. Conductometric, surface tension, and kinetic studies in mixed SDS-tween 20 and SDS-SB3-12 micellar solutions. Langmuir 2004, 20, 10858–10867. [Google Scholar] [CrossRef]
- Mahiuddin, S.; Zech, O.; Raith, S.; Touraud, D.; Kunz, W. Catanionic micelles as a model to mimic biological membranes in the presence of anesthetic alcohols. Langmuir 2009, 25, 12516–12521. [Google Scholar] [CrossRef]
- Garcia-Rio, L.; Mendez, M.; Paleo, A.R.; Sardina, F.J. New insights in cyclodextrin: surfactant mixed systems from the use of neutral and anionic cyclodextrin derivatives. J. Phys. Chem. B 2007, 111, 12756–12764. [Google Scholar] [CrossRef]
- Lopez-Cornejo, P. Personal communication. Universidad de Sevilla: Sevilla, Spain, 2010. [Google Scholar]
- Lee, E.; Kim, J.K. ; Lee, M. Tubular stacking of water-soluble toroids triggered by guest encapsulation. J. Am. Chem. Soc. 2009, 131, 18242–18243. [Google Scholar]
- Gust, D.; Moore, T.A.; Moore, A.L. Mimicking photosynthetic solar energy transduction. Acc. Chem. Res. 2001, 34, 40–48. [Google Scholar] [CrossRef]
- Clark, C.D.; Hoffman, M.Z. Effect of solution medium on the rate constants of excited-state electron-transfer quenching reactions of ruthenium(II)-diimine photosensitizers. Coord. Chem. Rev. 1997, 159, 359–373. [Google Scholar]
- Pattabiraman, M.; Kaanumalle, L.S.; Natarajan, A.; Ramamurthy, V. Regioselective photodimerization of cinnamic acids in water: templation with cucurbiturils. Langmuir 2006, 22, 7605–7609. [Google Scholar] [CrossRef]
- Bhattacharyya, K. Solvation dynamics and proton transfer in supramolecular assemblies. Acc. Chem. Res. 2003, 36, 95–101. [Google Scholar] [CrossRef]
- Kalyanasundaram, K. Photophysics of molecules in micelle-forming surfactant solutions. Chem. Soc. Rev. 1978, 7, 453–472. [Google Scholar]
- Ananthapadmanabhan, K.P.; Goddard, E.D.; Turro, N.J.; Kuo, P.L. Fluorescence probes for critical micelle concentration. Langmuir 1985, 1, 352–355. [Google Scholar] [CrossRef]
- Marchena, M.; Sanchez, F. Kinetic of photochemical reaction under restricted geometry conditions. Prog. Reac. Kinet. Mech. 2010, 35, 27–80. [Google Scholar] [CrossRef]
- Maiti, N.C.; Krishna, M.M.G.; Britto, P.J.; Periasamy, N. Fluorescence dynamics of dye probes in micelles. J. Phys. Chem. B 1997, 101, 11051–11060. [Google Scholar]
- Yoshida, N.; Takechi, M.; Asano, T.; Moroi, Y.; Humphry-Baker, R.; Grätzel, M. Thermodynamics and kinetics of solubilization of naphthalene into anionic micelles. Chem. Phys. Lett. 2000, 332, 265–270. [Google Scholar] [CrossRef]
- Infelta, P.P.; Gratzel, M. Statistics of solubilizate distribution and its application to pyrene fluorescence in micellar systems : Concise kinetic-model. J. Chem. Phys. 1979, 70, 179–186. [Google Scholar] [CrossRef]
- Maestri, M.; Infelta, P.P.; Gratzel, M. Kinetics of fast light-induced redox processes in micellar systems: Intra-micellar electron-transfer. J. Chem. Phys. 1978, 69, 1522–1526. [Google Scholar] [CrossRef]
- Barzykin, A.V.; Tachiya, M. Unified treatment of luminescence quenching kinetics in micelles with quencher migration on the basis of a generalized Smoluchowski approach. J. Phys. Chem. B 1998, 102, 1296–1300. [Google Scholar] [CrossRef]
- Almgren, M.; Grieser, F.; Thomas, J.K. Dynamic and static aspects of solubilization of neutral arenes in ionic micellar solutions. J. Am. Chem. Soc. 1979, 101, 279–291. [Google Scholar] [CrossRef]
- Almgren, M.; Lofroth, J.E.; Vanstam, J. Fluorescence decay kinetics in monodisperse confinements with exchange of probes and quenchers. J. Phys. Chem. 1986, 90, 4431–4437. [Google Scholar] [CrossRef]
- Quina, F.H.; Toscano, V.G. Photophenomena in surfactant media-quenching of a water-soluble fluorescence probe by iodide-ion in micellar solutions of sodium dodecyl-sulfate. J. Phys. Chem. 1977, 81, 1750–1754. [Google Scholar] [CrossRef]
- Doughert, S.J.; Berg, J.C. Distribution equilibria in micellar solutions. J. Colloid Interface Sci. 1974, 48, 110–121. [Google Scholar] [CrossRef]
- Rinco, O.; Nolet, M.C.; Ovans, R.; Bohne, C. Probing the binding dynamics to sodium cholate aggregates using naphthalene derivatives as guests. Photochem. Photobiol. Sci. 2003, 2, 1140–1151. [Google Scholar] [CrossRef]
- Turro, N.J.; Okubo, T.; Chung, C.J. Analysis of static and dynamic host-guest associations of detergents with cyclodextrins via photo-luminescence methods. J. Am. Chem. Soc. 1982, 104, 1789–1794. [Google Scholar] [CrossRef]
- Guo, X.; Xu, H.; Guo, R. Fluorescence quenching of anthracene by N,N-diethylaniline in the sodium dodecyl sulfate/benzyl alcohol/water system. J. Colloid Interface Sci. 2001, 240, 559–565. [Google Scholar] [CrossRef]
- Hackett, J.W.; Turro, C. Bimolecular electron transfer quenching of neutral *[Ru(phen)2bps] by 4,4 -diheptyl viologen in water and bound to SDS micelles. J. Phys. Chem. A 1998, 102, 5728–5733. [Google Scholar] [CrossRef]
- Dressick, W.J.; Hauenstein, B.L.; Demas, J.N.; Degraff, B.A. Electron-transfer quenching of ruthenium(II) photosensitizers by mercury(II) chlorides . 2. reactions in aqueous sodium lauryl sulfate micellar solutions. Inorg. Chem. 1984, 23, 1107–1113. [Google Scholar] [CrossRef]
- Weidemaier, K.; Fayer, M.D. Role of diffusion in photoinduced electron transfer on a micelle surface: Theoretical and monte carlo investigations. J. Phys. Chem. 1996, 100, 3767–3774. [Google Scholar] [CrossRef]
- Weidemaier, K.; Tavernier, H.L.; Fayer, M.D. Photoinduced electron transfer on the surfaces of micelles. J. Phys. Chem. B 1997, 101, 9352–9361. [Google Scholar] [CrossRef]
- Weidemaier, K.; Fayer, M.D. Photoinduced electron-transfer and geminate recombination on a micelle surface: Analytical theory and monte-carlo simulations. J. Chem. Phys. 1995, 102, 3820–3829. [Google Scholar] [CrossRef]
- Barzykin, A.V.; Seki, K.; Tachiya, M. Theory of diffusion-assisted reactions on micelle surfaces: photoinduced electron transfer followed by back transfer. J. Phys. Chem. B 1999, 103, 9156–9160. [Google Scholar] [CrossRef]
- Ranganathan, R.; Vautier-Giongo, C.; Bales, B.L. Toward a hydrodynamic description of bimolecular collisions in micelles. an experimental test of the effect of the nature of the quencher on the fluorescence quenching of pyrene in SDS micelles and in bulk liquids. J. Phys. Chem. B. 2003, 107, 10312–10318. [Google Scholar] [CrossRef]
- Ami, T.; Fujimoto, K. Fluorescence labeling of DNA based on photochemical ligation. Sci. Tech. Adv. Mat. 2006, 7, 249–254. [Google Scholar] [CrossRef]
- Morrison, M.E.; Dorfman, R.C.; Clendening, W.D.; Kiserow, D.J.; Rossky, P.J.; Webber, S.E. Quenching kinetics of anthracene covalently bound to a polyelectrolyte .1. effects of ionic-strength. J. Phys. Chem. 1994, 98, 5534–5540. [Google Scholar] [CrossRef]
- Chu, D.Y.; Thomas, J.K. Photophysical studies of a water-soluble copolymer of methacrylic-acid and 1-pyreneacrylic acid. Macromolecules 1984, 17, 2142–2147. [Google Scholar] [CrossRef]
- Turro, N.J.; Arora, K.S. Pyrene as a photophysical probe for intermolecular interactions of water-soluble polymers in dilute-solutions. Polymer 1986, 27, 783–796. [Google Scholar] [CrossRef]
- Arora, K.; Turro, N.J. Polyelectrolyte effects on the quenching of pyrene fluorescence in solutions of a pyrene substituted poly(acrylic acid). J. Pol. Sci. Part A-Polymer Chemistry 1987, 25, 259–269. [Google Scholar] [CrossRef]
- Turro, N.J.; Okubo, T.; Chung, C.J.; Emert, J.; Catena, R. Polyelectrolyte-enhanced excimer formation of bis(alpha-naphthylmethyl)ammonium chloride and (alpha-naphthylmethyl)ammonium chloride. J. Am. Chem. Soc. 1982, 104, 4799–4803. [Google Scholar] [CrossRef]
- Wensel, T.G.; Meares, C.F.; Vlachy, V.; Matthew, J.B. Distribution of ions around DNA, probed by energy-transfer. Proc. Natl. Acad. Sci. U. S. A. 1986, 83, 3267–3271. [Google Scholar] [CrossRef]
- Arkin, M.R.; Stemp, E.D.A.; Turro, C.; Turro, N.J.; Barton, J.K. Luminescence quenching in supramolecular systems: a comparison of DNA- and SDS micelle-mediated photoinduced electron transfer between metal complexes. J. Am. Chem. Soc. 1996, 118, 2267–2274. [Google Scholar]
- Fukuzumi, S.; Nishimine, M.; Ohkubo, K.; Tkachenko, N.V.; Lemmetyinen, H. Driving force dependence of photoinduced electron transfer dynamics of intercalated molecules in DNA. J. Phys. Chem. B 2003, 107, 12511–12518. [Google Scholar]
- Yorozu, T.; Hoshino, M.; Imamura, M.; Shizuka, H. Photo-excited inclusion complexes of beta-naphthol with alpha-cyclodextrin, beta-cyclodextrin, and gamma-cyclodextrin in aqueous-solutions. J. Phys. Chem. 1982, 86, 4422–4426. [Google Scholar] [CrossRef]
- Andrade-Dias, C.; Lima, S.; Teixeira-Dias, J.J.C.; Teixeira, J. Why do methylated and unsubstituted cyclodextrins interact so differently with sodium decanoate micelles in water? J. Phys. Chem B. 2008, 112, 15327–15332. [Google Scholar] [CrossRef]
- Hamai, S. Association of inclusion-compounds of beta-cyclodextrin in aqueous-solution. Bull. Chem. Soc. Jpn. 1982, 55, 2721–2729. [Google Scholar] [CrossRef]
- Nelson, G.; Warner, I.M. Fluorescence quenching studies of cyclodextrin complexes of pyrene and naphthalene in the presence of alcohols. J. Phys. Chem. 1990, 94, 576–581. [Google Scholar] [CrossRef]
- Kano, K.; Takenoshita, I.; Ogawa, T. 3 Component complexes of cycloheptaamylose- fluorescence quenching of pyrenes and naphthalenes in aqueous-media. Chem. Lett. 1980, 1035–1038. [Google Scholar]
- Rideout, D.C.; Breslow, R. Hydrophobic acceleration of diels-alder reactions. J. Am. Chem. Soc. 1980, 102, 7816–7817. [Google Scholar]
- Kobashi, H.; Takahashi, M.; Muramatsu, Y.; Morita, T. Effects of beta-cyclodextrin on fluorescence quenching of sodium 1-pyrenesulfonate by aniline in aqueous-media. Bull. Chem. Soc. Jpn. 1981, 54, 2815–2816. [Google Scholar] [CrossRef]
- Breslow, R.; Czarniecki, M.F.; Emert, J.; Hamaguchi, H. Improved acylation rates within cyclodextrin complexes from flexible capping of the cyclodextrin and from adjustment of the substrate geometry. J. Am. Chem. Soc. 1980, 102, 762–770. [Google Scholar]
- Kano, K.; Takenoshita, I.; Ogawa, T. Fluorescence quenching of pyrene and naphthalene in aqueous cyclodextrin solutions:Evidence of 3-component complex-formation. J. Phys. Chem. 1982, 86, 1833–1838. [Google Scholar] [CrossRef]
- Garcia-Ruiz, C.; Hu, X. S.; Ariese, F.; Gooijer, C. Enantioselective room temperature phosphorescence detection of non-phosphorescent analytes based on interaction with beta-cyclodextrin/1-bromonaphthalene complexes. Talanta 2005, 66, 634–640. [Google Scholar] [CrossRef] [Green Version]
- Fukuzumi, S.; Kotani, H.; Ohkubo, K.; Ogo, S.; Tkachenko, N.V.; Lemmetyinen, H. Electron-transfer state of 9-mesityl-10-methylacridinium ion with a much longer lifetime and higher energy than that of the natural photosynthetic reaction center. J. Am. Chem. Soc. 2004, 126, 1600–1601. [Google Scholar]
- Creutz, C. Nonadiabatic, short-range, intramolecular electron transfer from ruthenium(II) to cobalt(III) complexes. J. Phys. Chem. B. 2007, 111, 6713–6717. [Google Scholar] [CrossRef]
- Gust, D.; Moore, T.A.; Moore, A.L. Solar fuels via artificial photosynthesis. Acc. Chem. Res. 2009, 42, 1890–1898. [Google Scholar] [CrossRef]
- Choi, H.; Kang, S.O.; Ko, J.; Gao, G.; Kang, H.S.; Kang, M.S.; Nazeeruddin, M.K.; Gratzel, M. An efficient dye-sensitized solar cell with an organic sensitizer encapsulated in a cyclodextrin cavity. angew. Chem. Int. 2009, 48, 5938–5941. [Google Scholar] [CrossRef]
- Somorjai, G.A.; Frei, H.; Park, J.Y. Advancing the frontiers in nanocatalysis, biointerfaces, and renewable energy conversion by innovations of surface techniques. J. Am. Chem. Soc. 2009, 131, 16589–16605. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an Open Access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bernal, E.; Marchena, M.; Sánchez, F. Microheterogeneous Catalysis. Molecules 2010, 15, 4815-4874. https://doi.org/10.3390/molecules15074815
Bernal E, Marchena M, Sánchez F. Microheterogeneous Catalysis. Molecules. 2010; 15(7):4815-4874. https://doi.org/10.3390/molecules15074815
Chicago/Turabian StyleBernal, Eva, María Marchena, and Francisco Sánchez. 2010. "Microheterogeneous Catalysis" Molecules 15, no. 7: 4815-4874. https://doi.org/10.3390/molecules15074815