Characterization of Destrins with Different Dextrose Equivalents
Abstract
:1. Introduction
2. Results and Discussion
2.1. SEM of dextrins with different DE values

2.2. X-ray study of dextrins

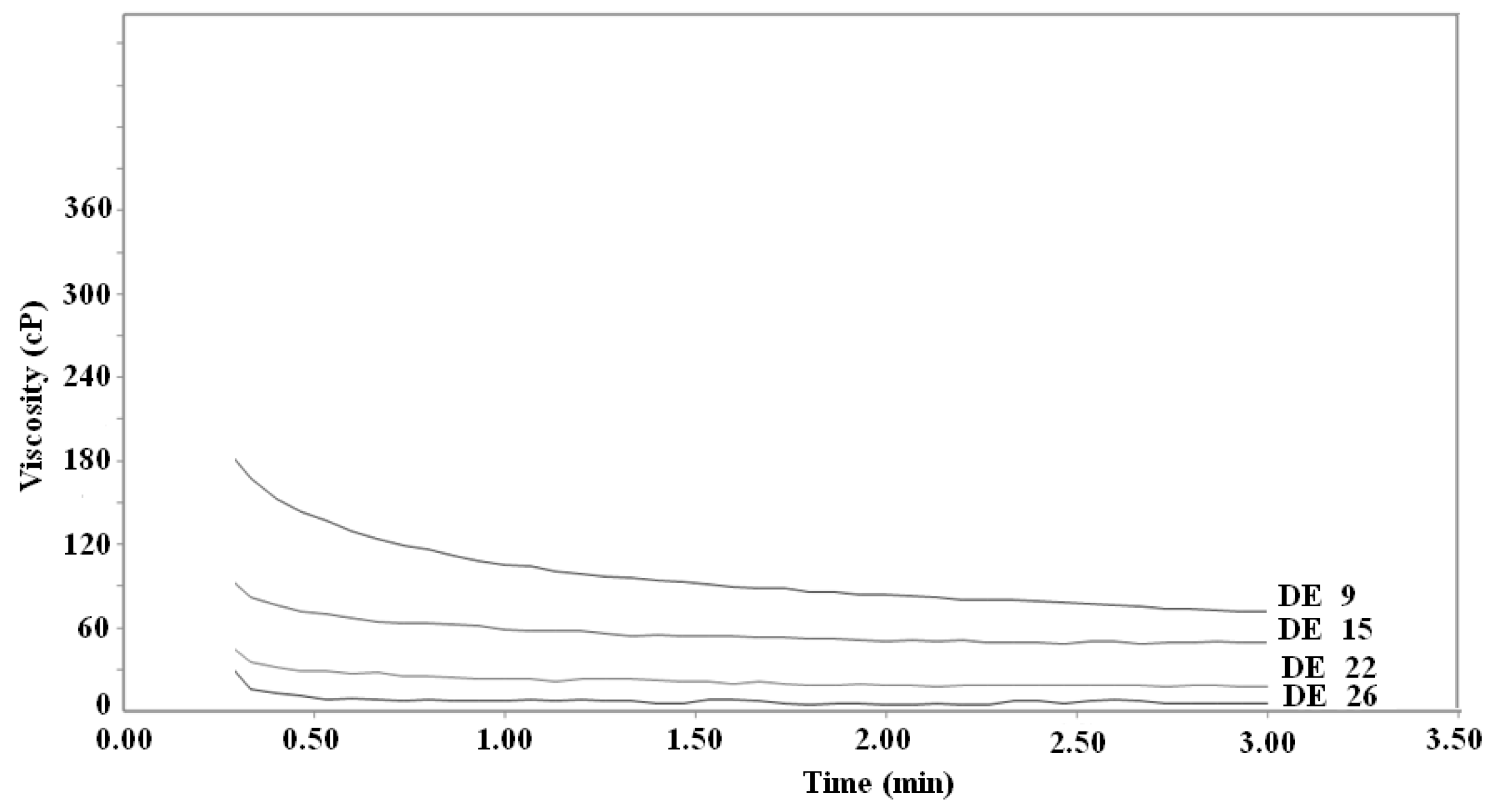
2.3. Viscosity of diluted solutions
2.4. Gel permeation chromatography

2.5. Molecular weight of dextrins

| Name | DE value | Retention Time (min) | Area | Area (%) | Mn | Mw | Mp | DPn | DPw |
|---|---|---|---|---|---|---|---|---|---|
| Peak 1 | 7 | 17.774 | 1297303 | 18.69 | 11132 | 15761 | 8041 | 68.7 | 97.3 |
| 9 | 17.467 | 934333 | 24.06 | 11751 | 17174 | 11472 | 72.5 | 106.0 | |
| 13 | 18.167 | 733533 | 16.84 | 10485 | 14810 | 5112 | 64.7 | 91.4 | |
| 15 | 18.317 | 757873 | 20.91 | 9290 | 13403 | 4299 | 57.3 | 82.7 | |
| 18 | 18.183 | 966087 | 20.19 | 10881 | 15825 | 5015 | 67.2 | 97.7 | |
| 22 | 17.547 | 870223 | 22.66 | 10346 | 15785 | 10453 | 63.9 | 97.4 | |
| 26 | 18.317 | 831988 | 15.98 | 8748 | 12776 | 4299 | 54.0 | 78.9 | |
| 28 | 17.456 | 876181 | 19.97 | 13824 | 19990 | 11616 | 85.3 | 123.4 | |
| Peak 2 | 7 | 19.647 | 5644866 | 81.31 | 633 | 1222 | 925 | 3.9 | 7.5 |
| 9 | 19.505 | 2948976 | 75.94 | 760 | 1383 | 1090 | 4.7 | 8.5 | |
| 13 | 19.616 | 3621699 | 83.16 | 732 | 1262 | 958 | 4.5 | 7.8 | |
| 15 | 19.459 | 2865852 | 79.09 | 788 | 1260 | 1149 | 4.9 | 7.8 | |
| 18 | 19.407 | 3817883 | 79.81 | 806 | 1344 | 1221 | 5.0 | 8.3 | |
| 22 | 19.360 | 2970766 | 77.34 | 783 | 1286 | 1288 | 4.8 | 7.9 | |
| 26 | 19.403 | 4375426 | 84.02 | 763 | 1248 | 1227 | 4.7 | 7.7 | |
| 28 | 19.282 | 3511781 | 80.03 | 876 | 1569 | 1410 | 5.4 | 9.7 |
2.6. Oligosaccharide composition of dextrins

| DE value | Area(% ) | |||
|---|---|---|---|---|
| G3 + G4 | G5 | G6 | DP≥ 7 | |
| 7 | 59.92 | 30.65 | 2.97 | 6.47 |
| 9 | 54.91 | 32.81 | 3.29 | 8.99 |
| 13 | 48.82 | 23.80 | 15.19 | 12.19 |
| 15 | 41.48 | 40.55 | 4.76 | 13.21 |
| 18 | 51.58 | 34.77 | 4.03 | 0.62 |
| 22 | 39.8 | 40.05 | 5.31 | 14.84 |
| 26 | 40.66 | 38.56 | 4.84 | 15.93 |
| 28 | 47.15 | 36.28 | 4.79 | 11.78 |
3. Experimental
3.1. Materials and reagents
3.2. Preparation of Dextrin
3.3. Determination of dextrin’s DE-value
3.4. Scanning electron micrographs of dextrin
3.5. X-ray diffractometry
3.6. Viscosity of dextrins
3.7. Gel permeation chromatography
3.8. Molecular weight determination of dextrins
3.9. Composition determination of dextrins with different DE value
4. Conclusions
- Sample Availability: Samples of the compounds are available from the authors.
References
- White, D.R., Jr.; Hudson, P.; Adamson, J.T. Dextrin characterization by high-performance anion-exchange chromatography-pulsed amperometric detection and size-exclusion chromatography–multi-angle light scattering-refractive index detection. J. Chromatogr. A 2003, 997, 79–85. [Google Scholar] [CrossRef]
- Tester, R.F.; Qi, X.; Karkalas, J. Hydrolysis of native starches with amylases. Anim. Feed Sci. Technol. 2006, 130, 39–54. [Google Scholar] [CrossRef]
- Chronakis, I.S.; Kasapis, S.; Richardson, R.K. Small deformation rheological properties of maltodextrin-milk protein systems. Carbohyd. Polym. 1996, 29, 137–148. [Google Scholar] [CrossRef]
- Lotfy, S. Controlling degradation of low-molecular-weight natural polymer “dextrin” using gamma irradiation. Int. J. Biol. Macromol. 2009, 44, 57–63. [Google Scholar] [CrossRef]
- Dokik, P.; Jakovljevic, J.; Dokic-Baucal, L. Molecular characteristics of maltodextrins and rheological behaviour of diluted and concentrated solutions. Colloids Surfaces A 1998, 141, 435–440. [Google Scholar] [CrossRef]
- Marchal, L.M.; Beeftink, H.H.; Tramper, J. Towards a rational design of commercial maltodextrins. Trends Food Sci. Technol. 1999, 10, 345–355. [Google Scholar] [CrossRef]
- MacGregor, E.A.; Janecek, S.; Svensson, B. Relationship of sequence and structure to specificity in the α-amylase family of enzymes. Biochim. Biophys. Acta 2001, 1546, 1–20. [Google Scholar] [CrossRef]
- Gupta, R.; Gigras, P.; Mohapatra, H.; Goswami, V.K.; Chauhan, B. Microbial α-amylases: a biotechnological perspective. Process Biochem. 2003, 38, 1599–1616. [Google Scholar] [CrossRef]
- Reilly, P.J. Protein engineering of glucoamylase to improve industrial performance—a review. Starch 1999, 51, 269–274. [Google Scholar] [CrossRef]
- Leveque, E.; Janecek, S.; Haye, B.; Belarbi, A. Thermophilic archaeal amylolytic enzymes. Enzyme Microb. Technol. 2000, 26, 3–14. [Google Scholar] [CrossRef]
- Slominska, L. Enzymatic Modification of Low Conversion Starch Products. Starch 1989, 41, 180–183. [Google Scholar] [CrossRef]
- Jane, J.L.; Wong, K.S.; McPherson, A.E. Branch-structure difference in starches of A- and B-type X-ray patterns revealed by their Naegeli dextrins. Carbohyd. Res. 1997, 300, 219–227. [Google Scholar] [CrossRef]
- Vermeylen, R.; Goderis, B.; Delcour, J.A. An X-ray study of hydrothermally treated potato starch. Carbohyd. Polym. 2006, 64, 364–375. [Google Scholar] [CrossRef]
- Liu, X.X.; Gu, Z.B.; Hong, Y. Composition and molecular distribution of maltodextrins and its effect on properties. Sci. Technol. Food Ind. 2006, 27, 97–100. [Google Scholar]
- Rani, S.; Bhattacharaya, K.R. Rheology of Rice-Flour Pastes: Effect of Variety, Concentration, and Temperature and Time of Cooking. J. Texture Stud. 1989, 20, 127–137. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an Open Access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sun, J.; Zhao, R.; Zeng, J.; Li, G.; Li, X. Characterization of Destrins with Different Dextrose Equivalents. Molecules 2010, 15, 5162-5173. https://doi.org/10.3390/molecules15085162
Sun J, Zhao R, Zeng J, Li G, Li X. Characterization of Destrins with Different Dextrose Equivalents. Molecules. 2010; 15(8):5162-5173. https://doi.org/10.3390/molecules15085162
Chicago/Turabian StyleSun, Junliang, Ruixiang Zhao, Jie Zeng, Guanglei Li, and Xinhua Li. 2010. "Characterization of Destrins with Different Dextrose Equivalents" Molecules 15, no. 8: 5162-5173. https://doi.org/10.3390/molecules15085162




