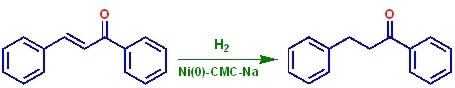
Ni(0)-CMC-Na Nickel Colloids in Sodium Carboxymethyl-Cellulose: Catalytic Evaluation in Hydrogenation Reactions
Abstract
:1. Introduction
2. Results and Discussion


| Entry | Polymer concentration g/L | Solvent | H2 pressure (bars) | Time (h) | Conversion % |
|---|---|---|---|---|---|
| 1 | 0.5 | H2O | 1 | 7 | 45 |
| 2 | 0.5 | H2O/iPrOH | 1 | 7 | 87 |
| 3 | 0.5 | H2O/CHCl3 | 1 | 24 | 5 |
| 4 | 0.5 | H2O/THF | 1 | 12 | 2 |
| 5 | 0.5 | H2O/Acetonitril | 1 | 16 | 5 |
| 6 | 0.5 | H2O/toluene | 1 | 24 | 0 |
| 7 | 0.5 | H2O/MeOH | 1 | 7 | 90 |
| 8 | 0.5 | H2O/MeOH | 10 | 5 | 91 |
| 9 | 0.5 | H2O/MeOH | 20 | 3.5 | 92 |
| 10 | 0.5 | H2O/MeOH | 40 | 2.0 | 98 |
| 11 | 1.0 | H2O/MeOH | 1 | 7 | 62 |
| 12 | 1.5 | H2O/MeOH | 1 | 7 | 57,5 |
| 13 | 2.0 | H2O/MeOH | 1 | 7 | 40 |
| 14 | 3.0 | H2O/MeOH | 1 | 7 | 12 |
| Entry | Reuses | Conversion % |
|---|---|---|
| 7 | 0 | 90 |
| 15 | 1 | 81 |
| 16 | 2 | 63 |
| 17 | 3 | 40 |
| Entry | Substrate | Product | Time (h) | Conversion % | Isolated yield % |
|---|---|---|---|---|---|
| 18 | 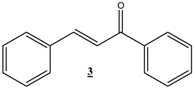 |  | 48 | 72 | 80 |
| 19 | 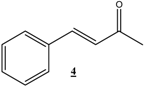 |  | 48 | 86 | 63 |
| 20 | 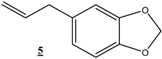 |  | 36 | 100 | 88 |
| 21 | 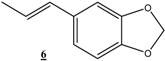 | 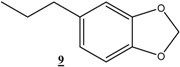 | 48 | 93 | 79 |
3. Experimental
3.1. Instrumentation
3.2. Preparation of the Metal Colloid Precursors
3.3. General Hydrogenation Procedure
3.4. Typical Spectral Data
4. Conclusions
References
- Lanone, S.; Boczkowski, J. Les sources de nanoparticules. Rev. Fran. Allergol. 2010, 50, 211–213. [Google Scholar]
- Prosie, F.; Lesage, F.X.; Deschamps, F. Nanoparticules: structures, utilisations et effets sur la santé. Press. Méd. 2008, 37, 1431–1437. [Google Scholar] [CrossRef]
- Tessonnier, J.P.; Pesant, L.; Ehret, G.; Ledoux, M.J.; Pham-Huu, C. Pd nanoparticles introduced inside multi-walled carbon nanotubes for selective hydrogenation of cinnamaldehyde into hydrocinnamaldehyde. Appl. Catal. A-Gen. 2005, 288, 203–210. [Google Scholar] [CrossRef]
- Leger, B.; Nowicki, A.; Roucoux, A.; Rolland, J.P. Competitive hydrogenation/dehalogenation of halogenoarenes with surfactant-stabilized aqueous suspensions of rhodium and palladium colloids: A major effect of the metal nature. J. Mol. Catal. A: Chem. 2007, 266, 221–225. [Google Scholar] [CrossRef]
- Guo, D.J.; Mei, H.H.; Wang, J.; Xiao, S.J.; Dai, Z.D. Surface-hydrophilic and protein-resistant silicone elastomers prepared by hydrosilylation of vinyl poly(ethylene glycol) on hydrosilanes-poly(dimethylsiloxane) surfaces. Colloid. Surface. A 2007, 308, 129–135. [Google Scholar] [CrossRef]
- Launay, F.; Roucoux, A.; Patin, H. Ruthenium colloids: A new catalyst for alkane oxidation by tBHP in a biphasic water-organic phase system. Tetrahedron Lett. 1998, 39, 1353–1356. [Google Scholar] [CrossRef]
- Jansat, S.; Picurelli, D.; Pelzer, K.; Philippot, K.; Gómez, M.; Muller, G.; Lecante, P.; Chaudret, B. Synthesis, characterization and catalytic reactivity of ruthenium nanoparticles stabilized by chiral N-donor ligands. New J. Chem. 2006, 1, 115–122. [Google Scholar]
- Nowicki, A.; Romagné, M.; Roucoux, A. N-(2-hydroxyethyl)ammonium derivatives as protective agents for Pd(0) nanocolloids and catalytic investigation in Suzuki reactions in aqueous media. Catal. Commun. 2008, 10, 68–70. [Google Scholar] [CrossRef]
- Boutros, M.; Launay, F.; Nowicki, A.; Onfroy, T.; Semmer, V.H.; Gedeon, A.R. Reduced forms of Rh(III) containing MCM-41 silicas as hydrogenation catalysts for arene derivatives. J. Mol. Catal. A: Chem. 2006, 259, 91–98. [Google Scholar] [CrossRef]
- Barthe, L.; Hemati, M.; Philippot, K.; Chaudret, B.; Denicourt-nowicki, A.; Roucoux, A. Rhodium colloidal suspension deposition on porous silica particles by dry impregnation: Study of the influence of the reaction conditions on nanoparticles location and dispersion and catalytic reactivity. Chem. Engineer. J. 2009, 151, 372–379. [Google Scholar] [CrossRef] [Green Version]
- Mévellec, V.; Mattioda, C.; Schulz, J.; Roucoux, A.; Rolland, J.P. Enantioselective hydrogenation of ethyl pyruvate in biphasic liquid-liquid media by reusable surfactant-stabilized aqueous suspensions of platinum nanoparticles. J. Catal. 2004, 225, 1–6. [Google Scholar] [CrossRef]
- Mevellec, V.; Roucoux, A. Nanoheterogeneous catalytic hydrogenation of N-, O- or S-heteroaromatic compounds by re-usable aqueous colloidal suspensions of rhodium(0). Inorg. Chim. Acta 2004, 357, 3099–3103. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Lin, K.-Y.; Tsai, W.-T.; Chang, J.-K.; Tseng, C.-M. Electroless Deposition of Ni nanoparticles on carbon nanotubes with the aid of supercritical CO2 fluid and a synergistic hydrogen storage property of the composite. Int. J. Hydrogen Energ. 2010, 35, 5490–5497. [Google Scholar] [CrossRef]
- Feygenson, M.; Kou, A.; Kreno, L.E.; Tiano, A.L.; Patete, J.M.; Zhang, F.; Sung Kim, M.; Solovyov, V.; Wong, S.S.; Aronson, M.C. Properties of highly crystalline NiO and Ni nanoparticles prepared by high-temperature oxidation and reduction. Phys. Rev. B 2010, 81. [Google Scholar] [PubMed]
- Kim, S.-G.; Terashi, Y.; Purwanto, A.; Okuyama, K. Synthesis and film deposition of Ni nanoparticles for base metal electrode applications. Colloid. Surf. A 2009, 337, 96–101. [Google Scholar] [CrossRef]
- Wei, Z.; Yan, P.; Feng, W.; Dai, J.; Wang, Q.; Xia, T. Microstructural characterization of Ni nanoparticles prepared by Anodic Arc Plasma. Mater. Charact. 2006, 57, 176–181. [Google Scholar] [CrossRef]
- Choi, H.; Veriansyah, B.; Kim, J.; Kim, J.-D.; Jeong Won Kang, J.-W. Continuous synthesis of metal nanoparticles in supercritical methanol. J. Supercrit. Fluids 2010, 52, 285–291. [Google Scholar] [CrossRef]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Harrad, M.A.; Valerga, P.; Puerta, M.C.; Houssini, I.; Ali, M.A.; Firdoussi, L.E.; Karim, A. Ni(0)-CMC-Na Nickel Colloids in Sodium Carboxymethyl-Cellulose: Catalytic Evaluation in Hydrogenation Reactions. Molecules 2011, 16, 367-372. https://doi.org/10.3390/molecules16010367
Harrad MA, Valerga P, Puerta MC, Houssini I, Ali MA, Firdoussi LE, Karim A. Ni(0)-CMC-Na Nickel Colloids in Sodium Carboxymethyl-Cellulose: Catalytic Evaluation in Hydrogenation Reactions. Molecules. 2011; 16(1):367-372. https://doi.org/10.3390/molecules16010367
Chicago/Turabian StyleHarrad, Mohamed Anouar, Pedro Valerga, M. Carmen Puerta, Issam Houssini, Mustapha Ait Ali, Larbi El Firdoussi, and Abdallah Karim. 2011. "Ni(0)-CMC-Na Nickel Colloids in Sodium Carboxymethyl-Cellulose: Catalytic Evaluation in Hydrogenation Reactions" Molecules 16, no. 1: 367-372. https://doi.org/10.3390/molecules16010367