SFE-CO2 Extract from Typhonium giganteum Engl. Tubers, Induces Apoptosis in Human Hepatoma SMMC-7721 Cells Involvement of a ROS-Mediated Mitochondrial Pathway
Abstract
:1. Introduction
2. Results and Discussion
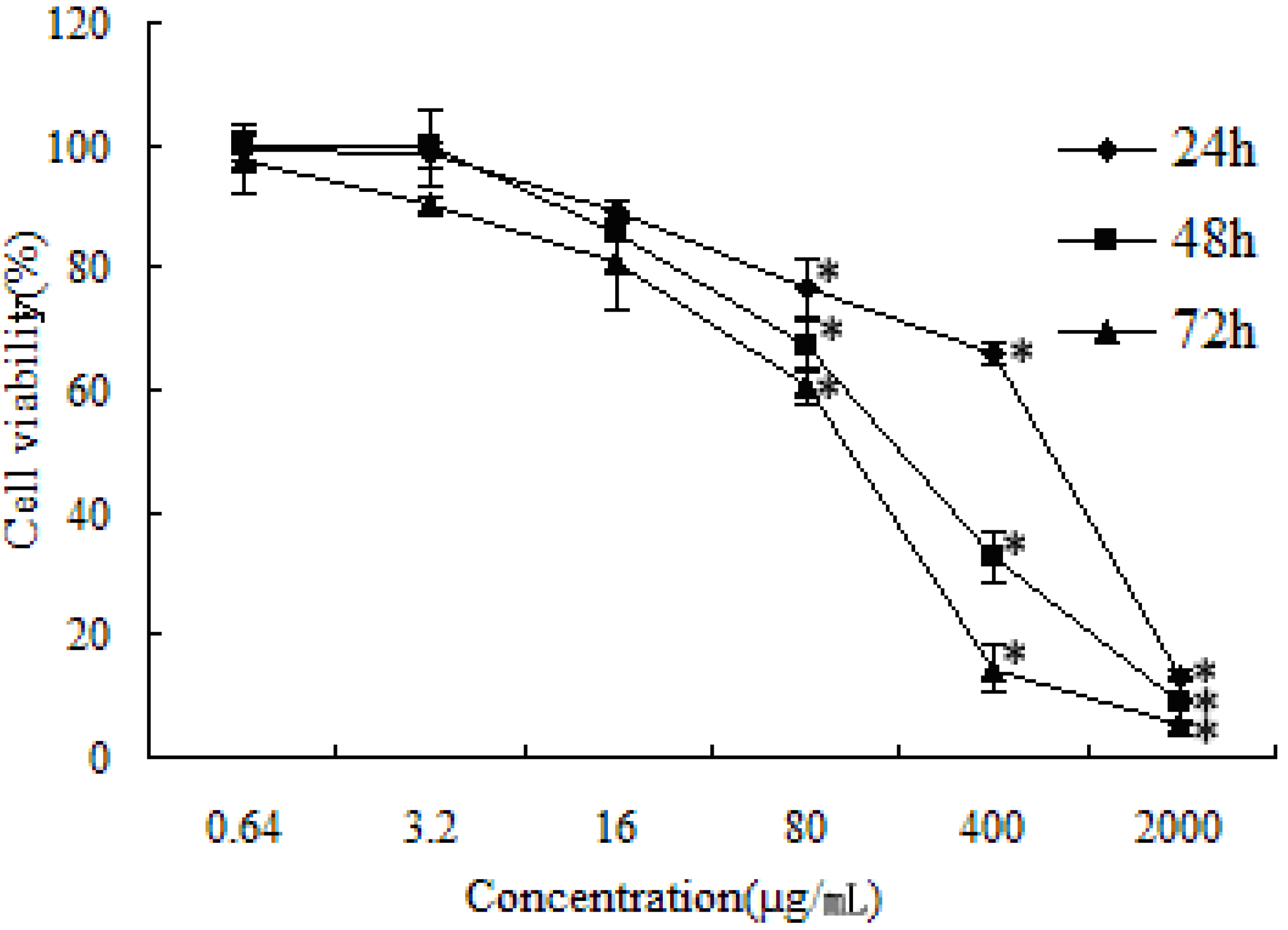
2.1. Cytotoxicity Assay
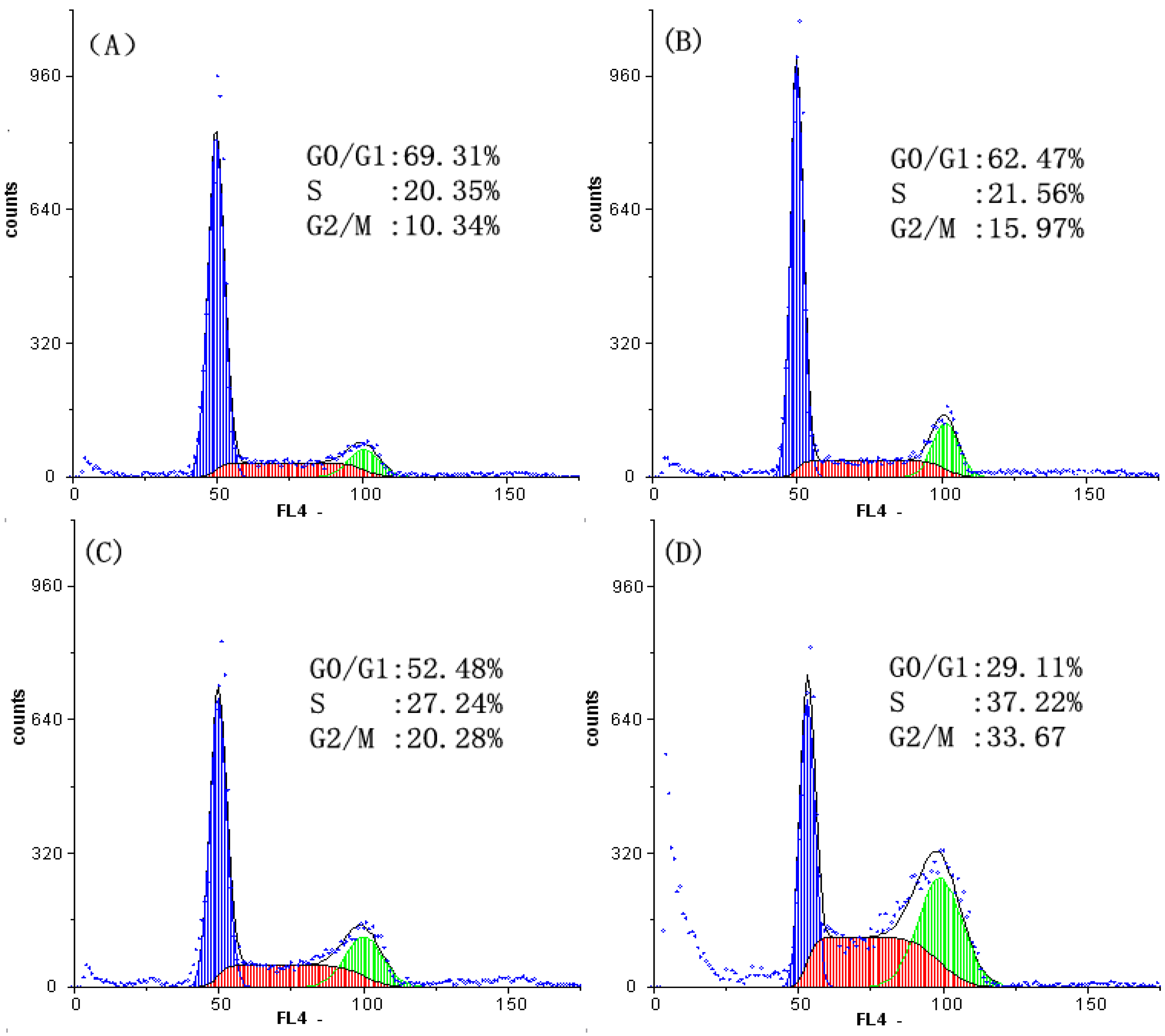
2.2. Analysis of Cell Cycle Distribution
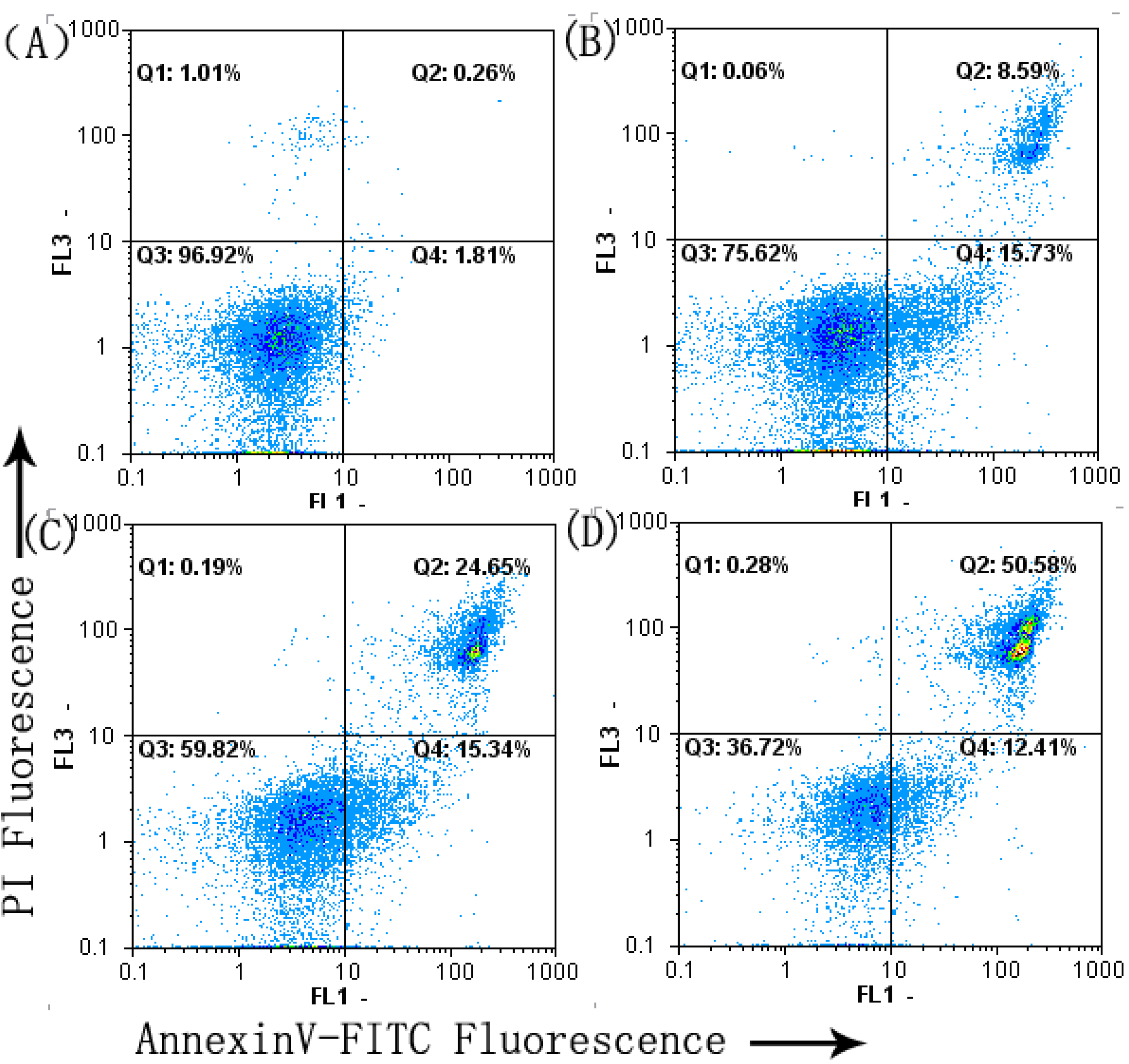
2.3. Cell Apoptosis Analysis
2.4. Changes in Nuclear Morphology

2.5. SFE-CO2 Extract Decreases Mitochondrial Membrane Potential( MMP) in SMMC-7721 Cells

2.6. Effect of SFE-CO2 Extract on Intracellular ROS in SMMC-7721 Cells

2.7. Effect of SFE-CO2 Extract on Intracellular Calcium Concentration ([Ca2+]c) in SMMC-7721 Cells
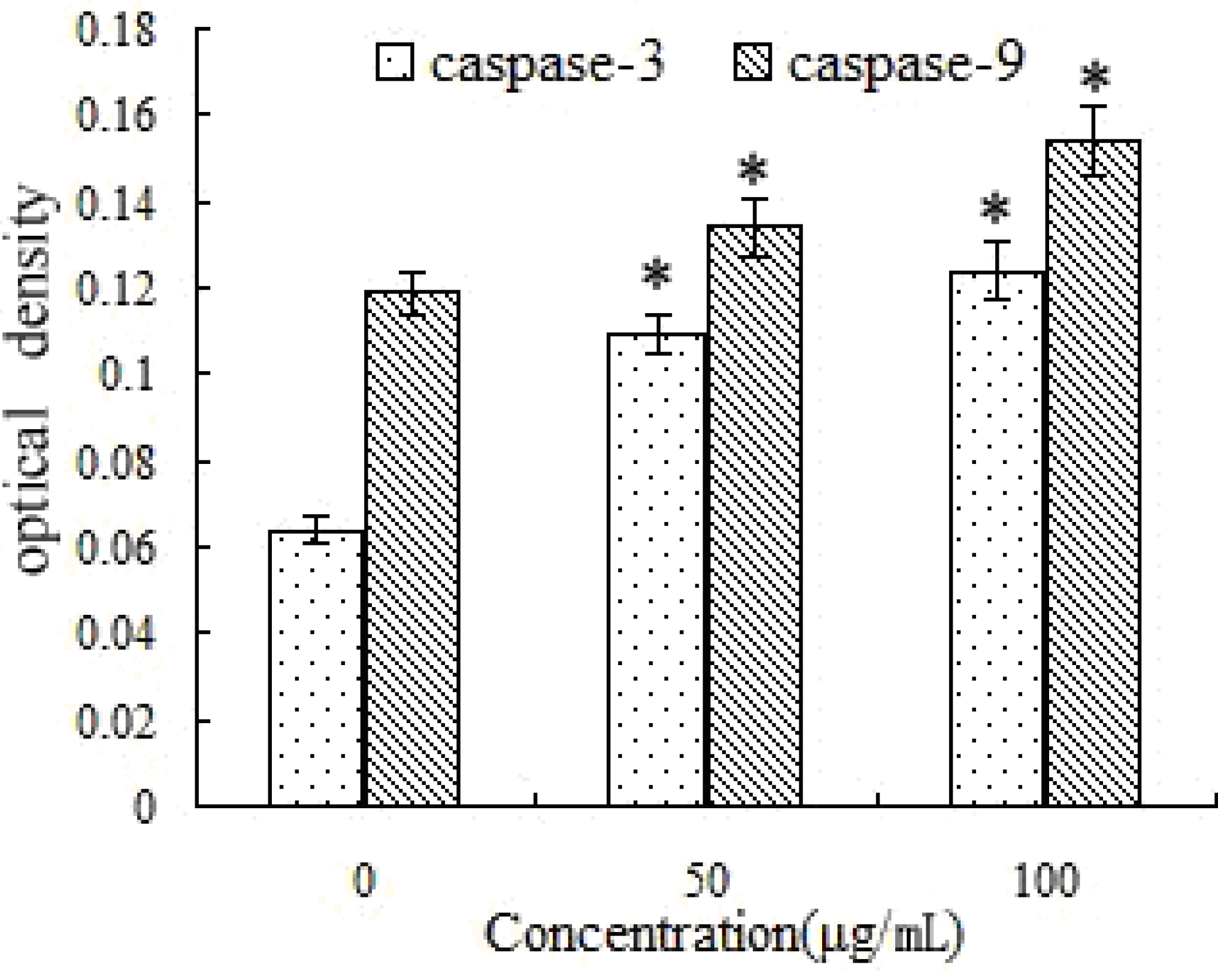
2.8. Activation of Caspase-3 and -9 by SFE-CO2 Extract

2.9. SFE-CO2 Extract-Mediated Up-Regulation of Bax and Down-Regulation of Bcl-2

3. Experimental
3.1. Plant Material and Extraction
3.2. Maintenance of Human Cancer Cell Lines
3.3. Cytotoxicity Assay

3.4. Cell Cycle Distribution
3.5. Apoptosis Assays
3.6. Nuclear Staining with Hoechst33258
3.7. Measurement of ROS Generation
3.8. The Changes of Mitochondrial Membrane Potential (MMP)
3.9. Intracellular Calcium Analysis
3.10. Measurement of Caspase-3 and Caspase-9 Activities
3.11. Western Blot Analysis
3.12. Statistical Analysis
4. Conclusions
Conflict of Interest
Acknowledgements
References
- Vinatier, D.; Dufour, Ph.; Subtil, D. Apoptosis: A programmed cell death involved in ovarian and uterine physiology. Eur. J. Obstet. Gynecol. Reprod. Biol. 1996, 67, 85–102. [Google Scholar] [CrossRef]
- Das, G.C.; Holiday, D.; Gallardo, R.; Haas, C. Taxol-induced cell cycle arrest and apoptosis: dose–response relationship in lung cancer cells of different wildtype p53 status and under isogenic condition. Cancer Lett. 2001, 165, 147–153. [Google Scholar] [CrossRef]
- Debatin, K.M. Activation of apoptosis pathways by anticancer treatment. Toxicol. Lett. 2000, 112-113, 41–48. [Google Scholar] [CrossRef]
- da Silva, C.P.; de Oliveira, C.R.; da Conceição, P.; de Lima, M. Apoptosis as a mechanism of cell death induced by different chemotherapeutic drugs in human leukemic T-lymphocytes. Biochem. Pharmacol. 1996, 51, 1331–134. [Google Scholar]
- Preston, T.J.; Abadi, A.; Wilson, L.; Singh, G. Mitochondrial contributions to cancer cell physiology: Potential for drug development. Adv. Drug Deliv. Rev. 2001, 49, 45–61. [Google Scholar] [CrossRef]
- Pulido, M.D.; Parrish, A.R. Metal-induced apoptosis: Mechanisms. Mutat. Res. 2003, 533, 227–241. [Google Scholar] [CrossRef]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688–694. [Google Scholar]
- Park, M.T.; Kim, M.J.; Kang, Y.H.; Choi, S.Y.; Lee, J.H.; Choi, J.A.; Kang, C.M.; Cho, C.K.; Kang, S.; Bae, S.; et al. Phytosphingosine in combination with ionizing radiation enhances apoptotic cell death in radiation-resistant cancer cells through ROS-dependent and -independent AIF release. Blood 2005, 105, 1724–1733. [Google Scholar] [CrossRef]
- Fiers, W.; Beyaert, R.; Declercq, W.; Vandenabeele, P. More than one way to die: Apoptosis, necrosis and reactive oxygen damage. Oncogene 1999, 18, 7719–7730. [Google Scholar]
- Galan, C.; Jardin, I.; Dionisio, N.; Salido, G.; Rosado, J.A. Role of Oxidant Scavengers in the Prevention of Ca2+ Homeostasis Disorders. Molecules 2010, 15, 7167–7187. [Google Scholar] [CrossRef]
- Reed, J.C. Apoptosis-regulating proteins as targets for drug discovery. Trends Mol. Med. 2001, 7, 314–319. [Google Scholar] [CrossRef]
- Lazebnik, Y.A.; Kaufmann, S.H.; Desnoyers, S.; Poirier, G.G.; Earnshaw, W.C. Cleavage of poly(ADP-ribose) polymerase by a proteinase with properties like ICE. Nature 1994, 371, 346–347. [Google Scholar]
- Chen, X.S.; Wu, Y.L.; Chen, D.H. Structure determination and synthesis of a new cerebroside isolated from traditional Chinese medicine Typhonium giganteum Engl. Tetrahedron Lett. 2002, 43, 3529–3532. [Google Scholar] [CrossRef]
- Zhu, T.; Wu, H.; Zhang, L. A Survey of Studies on Rhizoma Typhonii. Chin. Arch. Tradit. Chin. Med. 2008, 26, 1176–1178. [Google Scholar]
- Lee, C.H.; Chia, T.T. Chemical investigation of the rhizome of typhoninm giganteum engler. Yao Xue Xue Bao 1962, 9, 643–648. [Google Scholar]
- Chen, X.S.; Chen, D.H.; Si, J.Y.; Tu, G.Z. Chemical Constituents of Typhonium Giganteum Engl. J. Asian Nat. Prod. Res. 2001, 3, 277–283. [Google Scholar] [CrossRef]
- Li, J.; Wei, Y.; Chen, W.; Li, Y. Study on Chemical Constituents of Volatile Oil in the Rhizome of Typhonium Giganteum Engl. J. Jilin Agric. Univ. 1996, 18, 29–31. [Google Scholar]
- Hua, D.; Rui, K.; Gang, H.L.; Wang, Z.G. The Study on the Expression of HORN LIAN to Angiogenic Factor bFGF in the Anti-tumor Effect on H22 Tumor-bearing Mice. Inf. Tradit. Chin. Med. 2011, 28, 97–100. [Google Scholar]
- Wang, S.Q.; Ni, H.; Wang, J.; Chen, L. The inhibition effects of typhonium giganteum engl. on hepatocarcinoma cell. Chin. J. Cell Biol. 2003, 25, 185–188. [Google Scholar]
- Wang, L.M.; Ye, B.; Zhao, Z.J.; Li, S.Y. Inhibition of Proliferation and Induction of Apoptosis in MCF-7 Cell by Typhonium Giganteum Engl. J. Shenyang Agric. Univ. 2009, 40, 174–177. [Google Scholar]
- Wang, S.Q.; Ni, H.; Cheng, H.; Wang, G.L.; Wang, T.S.; Chen, L. Detection of differentially expressed genes in hepatocellularcarcinoma cells SMMC-7721 treated with Typhonium giganteum extract by mRNA differential display. Zhongguo Zhong Yao Za Zhi 2004, 29, 974–977. [Google Scholar]
- Li, Q.Y.; Wang, C.C.; Song, Z.; Qu, Z.H.; Jiang, C.F.; Qiu, W. GC-MS Analysis and in vitro antitumor activity of the extract from typhonium giganteum engl using supercritical fluid CO2. Bull. Bot. Res. 2011, 31, 113–116. [Google Scholar]
- Gschwind, M.; Huber, G. Apoptotic cell death induced by b-amyloid 1-42 peptide is cell type dependent. J. Neurochem. 1995, 65, 292–300. [Google Scholar] [CrossRef]
- Kroemer, G.; Galluzzi, L.; Brenner, C. Mitochondrial membrane permeabilization in cell death. Physiol. Rev. 2007, 87, 99–163. [Google Scholar] [CrossRef]
- Han, J.; Goldstein, L.A.; Gastman, B.R.; Rabinowich, H. Interrelated roles for Mcl-1 and BIM in regulation of TRAIL-mediated mitochondrial apoptosis. J. Biol. Chem. 2006, 281, 10153–10163. [Google Scholar]
- Fu, Y.R.; Yi, Z.J.; Yan, Y.R.; Qiu, Z.Y. Hydroxycamptothecin-induced apoptosis in hepatoma SMMC-7721 cells and the role of mitochondrial pathway. Mitochondrion 2006, 6, 211–217. [Google Scholar] [CrossRef]
- Kroemer, G.; Zamzami, N.; Susin, S. Mitochondrial control of apoptosis. Immunol. Today 1997, 18, 44–51. [Google Scholar] [CrossRef]
- Lluis, J.M.; Buricchi, F.; Chiarugi, P.; Morales, A.; Fernandez-Checa, J.C. Dual role of mitochondrial reactive oxygen species in hypoxia signaling: activation of nuclear factor-kB via c-SRC and oxidant-dependent cell death. Cancer Res. 2007, 67, 7368–7377. [Google Scholar] [CrossRef]
- Bhutia, S.K.; Mallick, S.K.; Maiti, S.; Mishra, D.; Maiti, T.K. Abrus abrin derived peptides induce apoptosis by targeting mitochondria in HeLa cells. Cell Biol. Int. 2009, 33, 720–727. [Google Scholar] [CrossRef]
- Lazebnik, Y.A.; Kaufmann, S.H.; Desnoyers, S.; Poirier, G.G.; Earnshaw, W.C. Cleavage of poly(ADP-ribose) polymerase by a proteinase with properties like ICE. Nature 1994, 371, 346–347. [Google Scholar]
- Han, S.I.; Kim, Y.S.; Kim, T.H. Role of apoptotic and necrotic cell death under physiologic conditions. BMB Rep. 2008, 41, 1–10. [Google Scholar]
- Thees, S.; Hubbard, G.B.; Winckler, J.; Schultz, C.; Rami, A. Specific alteration of the Bax/Bcl2 ratio and cytochrome c without execution of apoptosis in the hippocampus of aged baboons. Restor. Neurol. Neurosci. 2005, 23, 1–9. [Google Scholar]
- Zinkel, S.; Gross, A.; Yang, E. Bcl-2 family in DNA damage and cell cycle control. Cell Death Differ. 2006, 13, 1351–359. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Meth. 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Jiang, S.G.; Zu, Y.G.; Fu, Y.J.; Zhang, Y. Efferth, T. Activation of the mitochondriadriven pathway of apoptosis in human PC-3 prostate cancer cells by a novel hydrophilic paclitaxel derivative, 7-xylosyl-10-deacetylpaclitaxel. Int. J. Oncol. 2008, 33, 103–111. [Google Scholar]
- Han, L.L.; Xie, L.P.; Li, L.H.; Zhang, X.W.; Zhang, R.Q.; Wang, H.Z. Reactive oxygen species production and Bax/Bcl-2 regulation in honokiol-induced apoptosis in human hepatocellular carcinoma SMMC-7721 cells. Environ. Toxicol. Phar. 2009, 28, 97–103. [Google Scholar] [CrossRef]
- Cao, J.; Liu, Y.; Jia, L.; Zhou, H.M.; Kong, Y.; Yang, G.; Jiang, L.P.; Li, Q.J.; Zhong, L.F. Curcumin induces apoptosis through mitochondrial hyperpolarization and mtDNA damage in human hepatoma G2 cells. Free Radic. Biol. Med. 2007, 43, 968–975. [Google Scholar] [CrossRef]
- Zamzami, N.; Maisse, C.; Metivier, D.; Kroemer, G. Measurement of membrane permeability and permeability transition of mitochondria. Methods Cell Biol. 2001, 65, 147–158. [Google Scholar] [CrossRef]
- Burchiel, S.W.; Edwards, B.S.; Kuckuck, F.W.; Lauer, F.T.; Prossnitz, E.R.; Ransom, J.T.; Sklar, L.A. Analysis of free intracellular calcium by flow cytometry: multiparameter and pharmacologic applications. Methods 2000, 21, 221–230. [Google Scholar] [CrossRef]
- Wang, L.M.; Li, Q.Y.; Zu, Y.G.; Fu, Y.J.; Chen, L.Y.; Lv, H.Y.; Yao, L.P.; Jiang, S.G. Antiproliferative and pro-apoptotic effect of CPT13, a novel camptothecin analog, onhumancolon cancer HCT8 cell line. Chem. Biol. Interact. 2008, 176, 165–172. [Google Scholar] [CrossRef]
- Hsu, H.F.; Huang, K.H.; Lu, K.J.; Chiou, S.J.; Yen, J.H.; Chang, C.C.; Houng, J.Y. Typhonium blumei extract inhibits proliferation of human lung adenocarcinoma A549 cells via induction of cell cycle arrest and apoptosis. J. Ethnopharmacol. 2011, 135, 492–500. [Google Scholar] [CrossRef]
- Mohan, S.; Abdula, A.B.; Abdelwahaba, S.I.; Al-Zubairi, A.S.; Sukari, M.A.; Abdullah, R.; Taha, M.M.E.; Ibrahim, M.Y.; Syam, S. Typhonium flagelliforme induces apoptosis in CEMss cells via activation of caspase-9, PARP cleavage and cytochrome c release: Its activation coupled with G0/G1 phase cell cycle arrest. J. Ethnopharmacol. 2010, 131, 592–600. [Google Scholar] [CrossRef] [Green Version]
- Sample Availability: Samples of the compounds are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Li, Q.; Jiang, C.; Zu, Y.; Song, Z.; Zhang, B.; Meng, X.; Qiu, W.; Zhang, L. SFE-CO2 Extract from Typhonium giganteum Engl. Tubers, Induces Apoptosis in Human Hepatoma SMMC-7721 Cells Involvement of a ROS-Mediated Mitochondrial Pathway. Molecules 2011, 16, 8228-8243. https://doi.org/10.3390/molecules16108228
Li Q, Jiang C, Zu Y, Song Z, Zhang B, Meng X, Qiu W, Zhang L. SFE-CO2 Extract from Typhonium giganteum Engl. Tubers, Induces Apoptosis in Human Hepatoma SMMC-7721 Cells Involvement of a ROS-Mediated Mitochondrial Pathway. Molecules. 2011; 16(10):8228-8243. https://doi.org/10.3390/molecules16108228
Chicago/Turabian StyleLi, Qingyong, Chunfei Jiang, Yuangang Zu, Zhen Song, Baoyou Zhang, Xiangdong Meng, Wei Qiu, and Li Zhang. 2011. "SFE-CO2 Extract from Typhonium giganteum Engl. Tubers, Induces Apoptosis in Human Hepatoma SMMC-7721 Cells Involvement of a ROS-Mediated Mitochondrial Pathway" Molecules 16, no. 10: 8228-8243. https://doi.org/10.3390/molecules16108228



