Anti-Inflammatory Activity of Alkaloids: An Update from 2000 to 2010
Abstract
:1. Introduction
2. Results and Discussion
| Substance and (Source) | Assay | Organism tested | Dose | Activity | Ref. |
|---|---|---|---|---|---|
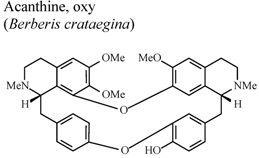 | In vivo, 5-HT-Induced pedal edema | Mouse | 200 mg/Kg | Inactive | [58] |
| In vivo, 5-HT-Induced pedal edema | Mouse | 200 mg/Kg | Active | [58] | |
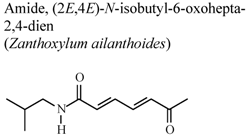
 | In vivo, inhibitory activity on superoxide generation by human neutrophils | Human | IC50 ≤ 5.34 µg/mL | Active | [67] |
| In vivo, inhibitory activity on elastase release by human neutrophils | Human | IC50 ≤ 5.53 µg/mL | Active | [67] | |
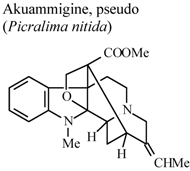 | In vivo, carrageenan-induced pedal edema | Rat | 1 mg/Kg | Active | [68] |
 | In vivo, inhibitory activity on superoxide generation by human neutrophils | Human | IC50 ≤ 5.34 µg/mL | Active | [67] |
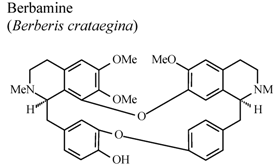 | In vivo, 5-HT-induced pedal edema | Mouse | 200 mg/Kg | Active | [58] |
| In vivo, 5-HT-induced pedal edema | Mouse | 100 mg/Kg | Active | [58] | |
| In vivo, TNB-induced colitis | Rat | 15 mg/Kg | Active | [69] | |
 | In vivo, LPS-induced hepatoxicity | Mouse | 100 mg/Kg | Inactive | [70] |
| In vivo, carrageenan-induced pedal edema | Mouse | 2 mg/Kg | Active | [70] | |
| In vivo, LPS-induced hepatoxicity | Mouse | 209 mg/Kg | Active | [70] | |
| In vivo, 5-HT induced-pedal edema | Mouse | 200 mg/Kg | Active | [58] | |
| In vivo, Carrageenan-induced pedal edema | Rat | 5 mg/Kg | Active | [55] | |
| In vivo, acute inflammation induced by E. coli LPS | Chicken | 15 mg/Kg | Active | [71] | |
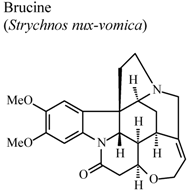 | In vivo, carrageenan-induced pedal edema | Rat | 15 mg/Kg | Active | [60] |
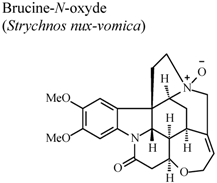 | In vivo, carrageenan-induced pedal edema | Rat | 100 mg/Kg | Active | [60] |
 | In vivo, capsaicin-induced ear edema | Mouse | 100 µmol/Kg | Active | [61] |
 | In humans, oral | Human adult | 0.5 mg/person | Active | [72] |
 | In vivo, External, 5-HT-induced pedal edema | Mouse | 200 mg/Kg | Inactive | [58] |
| In vivo, Intragastric, 5-HT-induced pedal edema | Mouse | 200 mg/Kg | Inactive | [58] | |
 | In vivo, inhibitory activity on superoxide generation by human neutrophils | Human | IC50 ≤ 5.34 µg/mL | Active | [67] |
| In vivo, inhibitory activity on elastase release by human neutrophils | Human | IC50 ≤ 5.53 µg/mL | Active | [67] | |
 | In vivo, carrageenan-induced paw edema | Rat | 10 mg/Kg | Active | [73] |
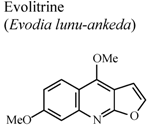 | In vivo, carrageenan-induced rat paw edema | Rat | 20 mg/Kg | Active | [74] |
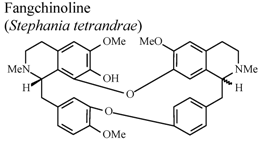 | In vivo, croton oil-induced edema | Mouse | 20 mg/Kg | Active | [75] |
| In vivo, croton oil-induced edema | Mouse | 0.1 mg/Kg | Active | [75] | |
| In vitro, fMLP-induced neutrophil adhesion and transmigration | Human | 10 µg/mL | Active | [76] | |
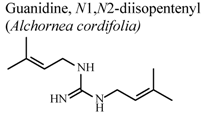 | In vivo, croton oil-induced ear edema | Mouse | 4.2 ± 0.5 mg/Kg | Active | [77] |
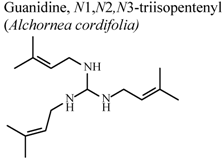 | In vivo, croton oil-induced ear edema | Mouse | 3.7 ± 0.8 mg/Kg | Active | [77] |
 | In vitro, inhibitory activity on superoxide anion generation | Human | IC50 < 5.5 5.43±1.52µg/mL | Active | [78] |
| In vitro, inhibitory activity on elastase release by human neutrophils | Human | IC50 < 5.5 3.25 ± 0.31 µg/mL | Active | [78] | |
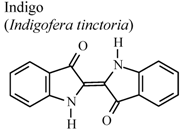 | In vivo, carrageenan-induced pedal edema | Mouse | 1 mg/Kg | Active | [79] |
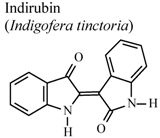 | In vivo, carrageenan-induced pedal edema | Mouse | 1 mg/Kg | Active | [79] |
 | * | * | * | Inactive | [80] |
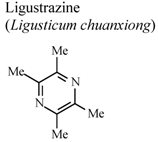 | In vitro, macrophages | Human adult | 400 mg/L | Active | [81] |
| In vivo, carrageenan-induced pedal edema | Rat | 50 mg/Kg | Active | [82] | |
| In vivo, Cotton pellet granuloma | Mouse | 50 mg/Kg | Active | [82] | |
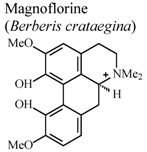 | In vivo, 5-HT-induced pedal edema | Mouse | 200 mg/Kg | Inactive | [58] |
| In vivo, 5-HT-induced pedal edema | Mouse | 200 mg/Kg | Inactive | [58] | |
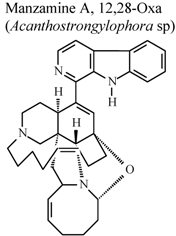 | * | * | * | Inactive | [80] |
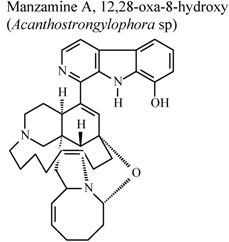 | * | * | * | Inactive | [80] |
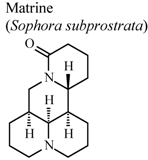 | In vitro, inhibitory activity of COX-1 | Rat | 31.3 µM | Active | [59] |
| In vitro, inhibitory activity of COX-2 | Rat | 188.5 µM | Moderate activity | [59] | |
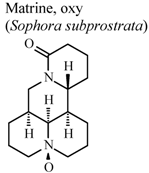 | In vitro, inhibitory activity of COX-1 | Rat | 197.8 µM | Moderate activity | [59] |
| In vitro, inhibitory activity of COX-2 | Rat | 385.1 µM | Weak activity | [59] | |
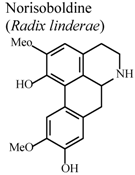 | In vivo, collagen II -induced arthritis | Mouse | 10 mg/Kg | Active | [83] |
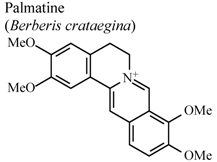 | In vivo, 5-HT-induced pedal edema | Mouse | 200 mg/Kg | Inactive | [58] |
| In vivo, 5-HT-induced pedal edema | Mouse | 100 mg/Kg | Active | [58] | |
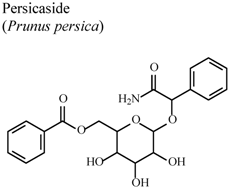 | In vitro, inhibitory activity on NO production | Rat | 40 µg/mL | Active | [84] |
| In vitro, inhibitory activity on PGE2 production | Rat | 40 µg/mL | Active | [84] | |
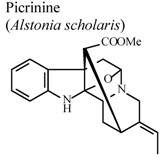 | In vitro, inhibitory activity on COX-1 | * | 100 µM | Inactive | [85] |
| In vitro, inhibitory activity on COX-2 | * | 100 µM | Weak activity | [85] | |
| In vitro, inhibitory activity on 5-LOX | * | 100 µM | Active | [85] | |
| In vivo, carrageenan-induced air pouch formation | Mouse | 10 mg/Kg | Active | [85] | |
| In vivo, xylene-induced ear edema | Mouse | 10 mg/Kg | Active | [85] | |
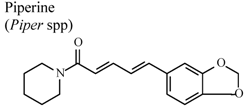 | In vivo, intragastric | Rat | 20 mg/Kg | Active | [86] |
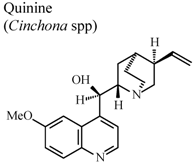 | In humans, oral | Human adult | 200 mg/day | Inactive | [87] |
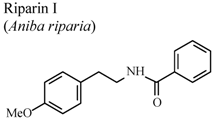 | In vivo, formalin test | Mice | 25 mg/Kg | Active | [88] |
| In vivo, carrageenan-induced pedal edema | Mice | 25 mg/Kg | Active | [65] | |
 | In vivo, carrageenan-induced pedal edema | Rat | 25 mg/Kg | Active | [66] |
 | In vitro, inhibitory activity on COX-1 | Mice | 100 µM | Active | [85] |
| In vitro, inhibitory activity on COX-2 | Mice | 100 µM | Active | [85] | |
| In vitro, inhibitory activity on 5-LOX | Mice | 100 µM | Active | [85] | |
| In vivo, carrageenan-induced air pouch formation | Mouse | 5 mg/Kg | Active | [85] | |
| In vivo, xylene-induced ear edema | Mouse | 5 mg/Kg | Active | [85] | |
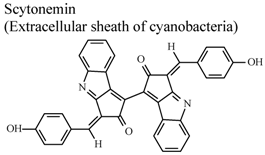 | In vitro, phorbol-induced edema of the mouse ear | Mouse | 5–100 µg/ear | Active | [89] |
 | In vivo, collagen II induced arthritis | Rat | 3.036 mg/Kg | Active | [90] |
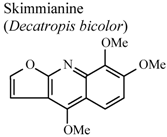 | In vivo, TPA-induced inflammation | Mouse | 0.75 mg/ear | Active | [91] |
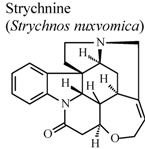 | In vivo, carrageenan-induced pedal edema | Rat | * | Inactive | [92] |
| In vivo, cotton pellet granuloma | Rat | * | Inactive | [92] | |
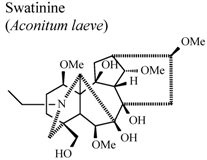 | In vitro, colorimetric assay with tetrazolium salt | Blood drawn from healthy volunteers | 100 µg/mL | Weak Activity | [93] |
 | In vivo, croton oil-induced edema | Mouse | 20 mg/Kg | Active | [75] |
| In vivo, croton oil-induced edema | Mouse | 0.1 mg/Kg | Active | [75] | |
| In vitro, FMLP-induced neutrophil adhesion and transmigration | Human | 10 µg/mL | Active | [76] | |
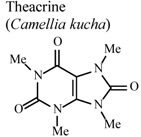 | In vivo, xylene-induced ear edema | Mouse | 8 mg/Kg | Active | [94] |
| In vivo, acetic acid-induced vascular permeability | Mouse | 16 mg/Kg | Active | [94] | |
| In vivo, carrageenan-induced paw edema | Mouse | 8 mg/Kg | Active | [94] | |
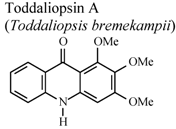 | In vitro, zymosan activated human polymorphonuclear leucocytes in a chemoluminescence assay system | Human | IC50 = 27.3 µg/mL | Weak activity | [95] |
 | In vitro, zymosan activated human polymorphonuclear leucocytes in a chemoluminescence assay system | Human | IC50 = 48.3 µg/mL | Weak activity | [95] |
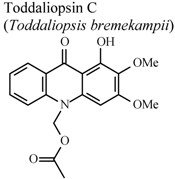 | In vitro, zymosan activated human polymorphonuclear leucocytes in a chemoluminescence assay system | Human | IC50 = 4.21 µg/mL | Active | [95] |
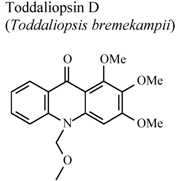 | In vitro, zymosan activated human polymorphonuclear leucocytes in a chemoluminescence assay system | Human | IC50 = 79.1 µg/mL | Weak activity | [95] |
 | In vitro, inhibitory activity on COX-1 | Mice | 100 µM | Active | [85] |
| In vitro, inhibitory activity on COX-2 | Mice | 100 µM | Active | [85] | |
| In vitro, inhibitory activity on 5-LOX | Mice | 100 µM | Active | [85] | |
| In vivo, carrageenan-induced air pouch formation | Mouse | 8 mg/Kg | Active | [85] | |
| In vivo, xylene-induced ear edema | Mouse | 8 mg/Kg | Active | [85] | |
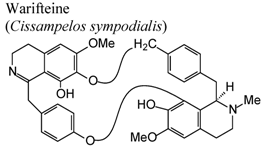 | In vivo, allergic eosinophilia and cysteinyl leukotrienes production | Mice | 50 μg/animal | Active | [56] |
| In vitro. OVA-sensitized animals were evaluated. The response was related with the increase of NO production | Mice | 0.4–10 mg/Kg | Active | [57] |
3. Conclusions
Acknowledgements
- Sample Availability: Samples of the compounds are not available from the authors.
References
- Vane, J.; Botting, R. Inflammation and the mechanism of action of anti-inflammatory drugs. FASEB J. 1987, 1, 89–96. [Google Scholar]
- Kulinsky, V.I. Biochemical aspects of inflammation. Biochemistry 2007, 72, 733–746. [Google Scholar]
- Blancas-Flores, G.; Almanza-Pérez, J.C.; López-Roa, R.I.; Alarcón-Aguilar, F.J.; García-Macedo, R.; Cruz, M. Obesity as an inflammatory process. Bol. Med. Hosp. Infant. Mex. 2010, 67, 88–96. [Google Scholar]
- Kim, S.; Choi, M.G.; Lee, H.S.; Lee, S.K.; Kim, S.H.; Kim, W.W.; Hur, S.M.; Kim, J.H.; Choe, J.H.; Nam, S.J.; et al. Silibinin suppresses TNF-α-induced MMP-9 expression in gastric cancer cells through Inhibition of the MAPK pathway. Molecules 2009, 14, 4300–4311. [Google Scholar] [CrossRef]
- Lee, D.C.W.; Lau, A.S.Y. Effects of Panax ginseng on tumor necrosis factor-α-mediated inflammation: a mini-review. Molecules 2011, 16, 2802–2816. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; He, T.; Han, Y.; Sheng, J.Z.; Jin, S.; Jin, M.W. Pentamethylquercetin improves adiponectin expression in differentiated 3T3-L1 cells via a mechanism that implicates PPARγ together with TNF-α and IL-6. Molecules 2011, 16, 5754–5768. [Google Scholar] [CrossRef]
- Qiao, Z.; Ma, J.; Liu, H. Effect of Ligusticum wallichii aqueous extract on oxidative injury and immunity activity in myocardial ischemic reperfusion rats. Int. J. Mol. Sci. 2011, 12, 1991–2006. [Google Scholar] [CrossRef]
- Ekstein, D.; Schachter, S.C. Natural products in epilepsy-the present situation and perspectives for the future. Pharmaceuticals 2010, 3, 1426–1445. [Google Scholar]
- Koehn, F.E.; Carter, G.T. The evolving role of natural products in drug discovery. Nature 2005, 4, 206–220. [Google Scholar]
- McCloud, T.G. High throughput extraction of plant, marine and fungal specimens for preservation of biologically active molecules. Molecules 2010, 15, 4526–4563. [Google Scholar] [CrossRef]
- Scotti, L.; Ferreira, E.I.; Silva, M.S.; Scotti, M.T. Chemometric studies on natural products as potential inhibitors of the NADH. Molecules 2010, 15, 7363–7377. [Google Scholar] [CrossRef]
- Reis, F.S.; Pereira, E.; Barros, L.; Sousa, M.J.; Martins, A.; Ferreira, I.C.F.R. Biomolecule profiles in inedible wild mushrooms with antioxidant value. Molecules 2011, 16, 4328–4338. [Google Scholar] [CrossRef]
- Kong, K.W.; Khoo, H.E.; Prasad, K.N.; Ismail, A.; Tan, C.P.; Rajab, N.F. Revealing the power of the natural red pigment lycopene. Molecules 2010, 15, 959–987. [Google Scholar] [CrossRef]
- Li, J.W.H.; Vederas, J.C. Drug discovery and natural products: End of an era or an endless frontier? Science 2009, 325, 161–165. [Google Scholar] [CrossRef]
- Cechinel Filho, V.; Yunes, R.A. Estratégias para obtenção de compostos farmacologicamente ativos a partir de plantas medicinais: Conceitos sobre modificação estrutural para otimização da atividade. Quim. Nova 1998, 21, 99–105. [Google Scholar] [CrossRef]
- Talib, W.H.; Mahasneh, A.M. Antimicrobial, cytotoxicity and phytochemical screening of Jordanian plants used in traditional medicine. Molecules 2010, 15, 1811–1824. [Google Scholar] [CrossRef]
- Henriques, A.T.; Limberger, R.P.; Kerber, V.A.; Moreno, P.R.H. Alcalóides: Generalidades e Aspectos Básicos. In Farmacognosia: Da Planta Ao Medicamento, 5th; Simões, C.M.O., Schenkel, E.P., Gosmann, G., Mello, J.C.P., Mentz, L.A., Petrovick, P.R., Eds.; Editoras of the Universidades Federais de Santa Catarina and Rio Grande do Sul: Porto Alegre/Florianópolis, Brazil, 2004; pp. 765–792, Chapter 29. [Google Scholar]
- Ezell, S.J.; Li, H.; Xu, H.; Zhang, X.; Gurpinar, E.; Zhang, X.; Rayburn, E.R.; Sommers, C.I.; Yang, X.; Velu, S.E.; et al. Preclinical pharmacology of BA-TPQ, a novel synthetic iminoquinone anticancer agent. Mar. Drugs 2010, 8, 2129–2141. [Google Scholar] [CrossRef]
- Aiello, A.; Fattorusso, E.; Imperatore, C.; Irace, C.; Luciano, P.; Menna, M.; Santamaria, R.; Vitalone, R. Zorrimidazolone, a bioactive alkaloid from the non-indigenous Mediterranean stolidobranch Polyandrocarpa zorritensis. Mar. Drugs 2011, 9, 1157–1165. [Google Scholar] [CrossRef] [Green Version]
- Falcão, H.S.; Leite, J.A.; Barbosa-Filho, J.M.; Athayde-Filho, P.F.; Chaves, M.C.O.; Moura, M.D.; Ferreira, A.L.; Almeida, A.B.A.; Souza-Brito, A.R.M.; Diniz, M.F.F.M.; et al. Gastric and duodenal antiulcer activity of alkaloids: A review. Molecules 2008, 13, 3198–3223. [Google Scholar] [CrossRef]
- Moura, M.D.; Torres, A.R.; Oliveira, R.A.G.; Diniz, M.F.F.M.; Barbosa-Filho, J.M. Natural products as inhibitors of models of mammary neoplasia. Br. J. Phytother. 2001, 5, 124–145. [Google Scholar]
- Moura, M.D.; Silva, J.S.; Oliveira, R.A.G.; Diniz, M.F.F.M.; Barbosa-Filho, J.M. Natural products reported as potential inhibitors of uterine cervical neoplasia. Acta Farm. Bonaer. 2002, 21, 67–74. [Google Scholar]
- Silva, J.S.; Moura, M.D.; Oliveira, R.A.G.; Diniz, M.F.F.M.; Barbosa-Filho, J.M. Natural product inhibitors of ovarian neoplasia. Phytomedicine 2003, 10, 221–232. [Google Scholar] [CrossRef]
- Gonçalves, M.C.R.; Moura, L.S.A.; Rabelo, L.A.; Barbosa-Filho, J.M.; Cruz, H.M.M.; Cruz, J. Natural products inhibitors of HMG CoA reductase. Rev. Bras. Farm. 2000, 81, 6371. [Google Scholar]
- Almeida, R.N.; Navarro, D.S.; Barbosa-Filho, J.M. Plants with central analgesic activity. Phytomedicine 2001, 8, 310–322. [Google Scholar] [CrossRef]
- Pereira, J.V.; Modesto-Filho, J.; Agra, M.F.; Barbosa-Filho, J.M. Plant and plant-derived compounds employed in prevention of the osteoporosis. Acta Farm. Bonaer. 2002, 21, 223–234. [Google Scholar]
- Morais, L.C.S.L.; Barbosa-Filho, J.M.; Almeida, R.N. Plants and bioactive compounds for the treatment of Parkinson’s disease. Arq. Bras. Fitomed.Cient. 2003, 1, 127–132. [Google Scholar]
- Rocha, L.G.; Almeida, J.R.G.S.; Macedo, R.O.; Barbosa-Filho, J.M. A review of natural products with antileishmanial activity. Phytomedicine 2005, 12, 514–535. [Google Scholar] [CrossRef]
- Barbosa-Filho, J.M.; Vasconcelos, T.H.C.; Alencar, A.A.; Batista, L.M.; Oliveira, R.A.G.; Guedes, D.N.; Falcão, H.S.; Moura, M.D.; Diniz, M.F.F.M.; Modesto-Filho, J. Plants and their active constituents from South, Central, and North America with hypoglycemic activity. Rev. Bras. Farmacogn. 2005, 15, 392–413. [Google Scholar] [CrossRef]
- Falcão, H.S.; Lima, I.O.; Santos, V.L.; Dantas, H.F.; Diniz, M.F.F.M.; Barbosa-Filho, J.M.; Batista, L.M. Review of the plants with anti-inflammatory activity studied in Brazil. Rev. Bras. Farmacogn. 2005, 15, 381–391. [Google Scholar] [CrossRef]
- Lima, G.R.M.; Montenegro, C.A.; Almeida, C.L.F.; Athayde-Filho, P.F.; Barbosa-Filho, J.M.; Batista, L.M. Database survey of anti-inflammatory plants in South America: A review. Int. J. Mol. Sci. 2011, 12, 2692–2749. [Google Scholar] [CrossRef]
- Barbosa-Filho, J.M.; Medeiros, K.C.P.; Diniz, M.F.F.M.; Batista, L.M.; Athayde-Filho, P.F.; Silva, M.S.; Cunha, E.V.L.; Almeida, J.R.G.S.; Quintans-Júnior, L.J. Natural products inhibitors of the enzyme acetylcholinesterase. Rev. Bras. Farmacogn. 2006, 16, 258–285. [Google Scholar] [CrossRef]
- Barbosa-Filho, J.M.; Martins, V.K.M.; Rabelo, L.A.; Moura, M.D.; Silva, M.S.; Cunha, E.V.L.; Souza, M.F.V.; Almeida, R.N.; Medeiros, I.A. Natural products inhibitors of the angiotensin converting enzyme (ACE). A review between 1980-2000. Rev. Bras. Farmacogn. 2006, 16, 421–446. [Google Scholar] [CrossRef]
- Amaral, F.M.M.; Ribeiro, M.N.S.; Barbosa-Filho, J.M.; Reis, A.S.; Nascimento, F.R.F.; Macedo, R.O. Plants and chemical constituents with giardicidal activity. Rev. Bras. Farmacogn. 2006, 16, 696–720. [Google Scholar] [CrossRef]
- Barbosa-Filho, J.M.; Nascimento-Júnior, F.A.; Tomaz, A.C.A.; Athayde-Filho, P.F.; Silva, M.S.; Cunha, E.V.L. Natural products with antileprotic activity. Rev. Bras. Farmacogn. 2007, 17, 141–148. [Google Scholar] [CrossRef]
- Falcão, H.S.; Mariath, I.R.; Diniz, M.F.F.M.; Batista, L.M.; Barbosa-Filho, J.M. Plants of the American continent with antiulcer activity. Phytomedicine 2008, 15, 132–146. [Google Scholar] [CrossRef]
- Mota, K.S.L.; Dias, G.E.N.; Pinto, M.E.F.; Luiz-Ferreira, A.; Souza-Brito, A.R.M.; Hiruma-Lima, C.A.; Barbosa-Filho, J.M.; Batista, L.M. Flavonoids with gastroprotective activity. Molecules 2009, 14, 979–1012. [Google Scholar]
- Agra, M.F.; França, P.F.; Barbosa-Filho, J.M. Synopsis of the plants known as medicinal and poisonous in Northeast of Brazil. Rev. Bras Farmacogn. 2007, 17, 114–140. [Google Scholar] [CrossRef]
- Agra, M.F.; Silva, K.N.; Basílio, I.J.L.D.; França, P.F.; Barbosa-Filho, J.M. Survey of medicinal plants used in the region Northeast of Brazil. Rev. Bras Farmacogn. 2008, 18, 472–508. [Google Scholar] [CrossRef]
- Silva, F.L.; Fischer, D.C.H.; Tavares, J.F.; Silva, M.S.; Athayde-Filho, P.F.; Barbosa-Filho, J.M. Compilation of secondary metabolites from Bidens pilosa L. Molecules 2011, 16, 1070–1102. [Google Scholar] [CrossRef]
- Quintans-Júnior, L.J.; Almeida, J.R.G.S.; Lima, J.T.; Nunes, X.P.; Siqueira, J.S.; Oliveira, L.E.G.; Almeida, R.N.; Athayde-Filho, P.F.; Barbosa-Filho, J.M. Plants with anticonvulsant properties-A review. Rev. Bras. Farmacogn. 2008, 18, 798–819. [Google Scholar] [CrossRef]
- Sousa, F.C.F.; Melo, C.T.V.; Citó, M.C.O.; Félix, F.H.C.; Vasconcelos, S.M.M.; Fonteles, M.M.F.; Barbosa-Filho, J.M.; Viana, G.S.B. Plantas medicinais e seus constituintes bioativos: Uma revisão da bioatividade e potenciais benefícios nos distúrbios da ansiedade em modelos animais. Rev. Bras. Farmacogn. 2008, 18, 642–654. [Google Scholar] [CrossRef]
- Ribeiro-Filho, J.; Falcão, H.S.; Batista, L.M.; Barbosa-Filho, J.M.; Piuvezam, M.R. Effects of plant extracts on HIV-1 protease. Curr. HIV Res. 2010, 8, 531–544. [Google Scholar] [CrossRef]
- Barbosa-Filho, J.M.; Alencar, A.A.; Nunes, X.P.; Tomaz, A.C.A.; Sena-Filho, J.G.; Athayde-Filho, P.F.; Silva, M.S.; Souza, M.F.V.; Cunha, E.V.L. Sources of alpha-, beta-, gamma-, delta- and epsilon-carotenes: A twentieth century review. Rev. Bras. Farmacogn. 2008, 18, 135–154. [Google Scholar] [CrossRef]
- Alves, J.S.; Castro, J.C.; Freire, M.O.; Cunha, E.V.L.; Barbosa-Filho, J.M.; Silva, M.S. Complete assignment of the 1H and 13C spectra of four triterpenes of the ursane, artane, lupane and friedelane groups. Magn. Reson. Chem. 2000, 38, 201–206. [Google Scholar] [CrossRef]
- Sena-Filho, J.G.; Duringer, J.M.; Maia, G.L.A.; Tavares, J.F.; Xavier, H.S.; Silva, M.S.; Cunha, E.V.L.; Barbosa-Filho, J.M. Ecdysteroids from Vitex species: Distribution and compilation of their 13C-NMR spectral data. Chem. Biodivers. 2008, 5, 707–713. [Google Scholar] [CrossRef]
- Oliveira, S.L.; Silva, M.S.; Tavares, J.F.; Sena-Filho, J.G.; Lucena, H.F.S.; Romero, M.A.V.; Barbosa-Filho, J.M. Tropane alkaloids from genus Erythroxylum: Distribution and compilation of 13C-NMR spectral data. Chem. Biodivers. 2010, 7, 302–326. [Google Scholar] [CrossRef]
- Vasconcelos, S.M.M.; Honório-Júnior, J.E.R.; Abreu, R.N.D.C.; Silva, M.C.C.; Barbosa-Filho, J.M.; Lobato, R.F.G. Pharmacologic Study of Some Plant Species from the Brazilian Northeast: Calotropis procera, Agava sisalana, Solanum paludosum, Dioscorea cayenensis and Crotalaria retusa. In Medicinal Plants: Classification, Biosynthesis and Pharmacology; Varela, A., Jasiah Ibañez, J., Eds.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2009; Volume 4, pp. 189–202. [Google Scholar]
- Vasconcelos, S.M.M.; Pereira, E.C.; Chaves, E.M.C.; Lobato, R.F.G.; Barbosa-Filho, J.M.; Patrocínio, M.C.A. Pharmacologic Study of Amburana cearensis and Aniba Genus. In Recent Progress in Medicinal Plants. Drug Plant IV; Singh, V.K., Govil, J.N., Eds.; Studium Press LLC: Houston, TX, USA, 2010; Volume 30, pp. 51–64. [Google Scholar]
- Almeida, C.L.F.; Falcão, H.S.; Lima, G.R.M.; Montenegro, C.A.; Lira, N.S.; Athayde-Filho, P.F.; Rodrigues, L.C.; Souza, M.F.V.; Barbosa-Filho, J.M.; Batista, L.M. Bioactivities from marine algae of the genus Gracilaria. Int. J. Mol. Sci. 2011, 12, 4550–4573. [Google Scholar]
- Barbosa-Filho, J.M.; Sette, I.M.F.; Cunha, E.V.L.; Guedes, D.N.; Silva, M.S. Protoberberine Alkaloids. In The Alkaloids; Cordell, G.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2005; Volume 62, pp. 1–75. [Google Scholar]
- Conserva, L.M.; Pereira, C.A.B.; Barbosa-Filho, J.M. Alkaloids of the Hernandiaceae: Occurrence and a Compilation of Their Biological Activities. In The Alkaloids; Cordell, G.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2005; Volume 62, pp. 175–243. [Google Scholar]
- Barbosa-Filho, J.M.; Piuvezam, M.R.; Moura, M.D.; Silva, M.S.; Lima, K.V.B.; da Cunha, E.V.L.; Fechine, I.M.; Takemura, O.S. Anti-inflammatory activity of alkaloids: A twenty-century review. Rev. Bras. Farmacogn. 2006, 16, 109–139. [Google Scholar] [CrossRef]
- Yoo, K.Y.; Hwang, I.K.; Kim, J.D.; Kang, I.J.; Park, J.; Yi, J.S.; Kim, J.K.; Bae, Y.S.; Won, M.H. Anti-inflammatory effect of the ethanol extract of Berberis koreana in a gerbil model of cerebral ischemia/reperfusion. Phytother. Res. 2008, 22, 1527–1532. [Google Scholar] [CrossRef]
- Kuo, C.L.; Chi, C.W.; Liu, T.Y. The anti-inflammatory potential of berberine in vitro and in vivo. Cancer Lett. 2004, 203, 127–137. [Google Scholar] [CrossRef]
- Bezerra-Santos, C.R.; Vieira-de-Abreu, A.; Barbosa-Filho, J.M.; Bandeira-Melo, C.; Piuvezam, M.R.; Bozza, P.T. Anti-allergic properties of Cissampelos sympodialis and its isolated alkaloid warifteine. Int. Immunopharmacol. 2006, 6, 1152–1160. [Google Scholar] [CrossRef]
- Costa, H.F.; Bezerra-Santos, C.R.; Barbosa Filho, J.M.; Martins, M.A.; Piuvezam, M.R. Warifteine, a bisbenzylisoquinoline alkaloid, decreases immediate allergic and thermal hyperalgesic reactions in sensitized animals. Int. Immunopharmacol. 2008, 8, 519–525. [Google Scholar] [CrossRef]
- Kupeli, E.; Kosar, M.; Yesilada, E.; Baser, K.H.C; Baser, C. A comparative study on the anti-inflammatory, antinociceptive and antipyretic effects of isoquinoline alkaloids from the roots of turkish berberis species. Life Sci. 2002, 72, 645–657. [Google Scholar] [CrossRef]
- Ao, C.W.; Araki, N.; Tawata, S. Cyclooxygenase inhibitory compounds with antioxidant activities from Sophora subprostrata. Asian J. Chem. 2009, 21, 745–754. [Google Scholar]
- Yin, W.; Wang, T.S.; Yin, F.Z.; Cai, B.C. Analgesic and anti-inflammatory properties of brucine and brucine-N-oxide extracted from seeds of Strychnos nuxvomica. J. Ethnopharmacol. 2003, 88, 205–214. [Google Scholar] [CrossRef]
- Souza, E.T.; Lira, D.P.; Queiroz, A.C.; Silva, D.J.C.; Aquino, A.B.; Mella, E.A.C.; Lorenzo, V.P.; Miranda, G.E.C.; Araújo-Júnior, J.X.; Chaves, M.C.O.; et al. The antinociceptive and anti-inflammatory activities of caulerpin, a bisindole alkaloid isolated from seaweed of the genus Caulerpa. Mar. Drugs 2009, 7, 689–704. [Google Scholar] [CrossRef]
- Alarif, W.M.; Abou-Elnaga, Z.S.; Ayyad, S.E.N.; Al-Lihaibi, S.S. Insecticidal metabolites from the green alga Caulerpa racemosa. Clean-Soil Air Water 2010, 38, 548–557. [Google Scholar] [CrossRef]
- Liu, Y.; Morgan, J.B.; Coothankandaswamy, V.; Liu, R.; Jekabson, M.B.; Mahdi, F.; Nagle, D.G.; Zhou, Y.D. The Caulerpa pigment caulerpin inhibits HIF-1 activation and mitochondrial respiration. J. Nat. Prod. 2009, 72, 2104–2109. [Google Scholar]
- Ayyad, S.E.N.; Badria, F.A. Caulerpine: An antitumor indole alkaloid from Caulerpa racemosa. Alex. J. Pharm. Sci. 1994, 8, 217–219. [Google Scholar]
- Leite, C.P.; Araújo, F.L.O.; Melo, C.T.V.; Gutierrez, S.J.C.; Barbosa-Filho, J.M.; Sousa, F.C.F. Anti-inflammatory activity of riparin I (O-methyl-N-benzoyl tyramine) on paw edema models in mice. Inflamm. Res. 2011, 60, 202. [Google Scholar]
- Sousa, F.C.F.; Carvalho, A.M.R.; Leite, C.P.; Rocha, N.F.M.; Rios, E.R.V.; Vasconcelos, L.F.; Melo, C.T.V.; Lima, S.T.; Barbosa-Filho, J.M.; Vasconcelos, S.M.M. Anti-inflammatory activity of riparin II (N-2-hydroxybenzoyl tyramine) in rats. Inflammat. Res. 2011, 60, 206. [Google Scholar]
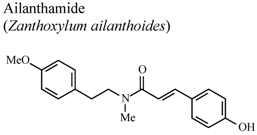
- Chen, J.J.; Chung, C.Y.; Hwang, T.L.; Chen, J.F. Amides and benzenoids from Zanthoxylum ailanthoides with inhibitory activity on superoxide generation and elastase release by neutrophils. J. Nat. Prod. 2009, 72, 107–111. [Google Scholar] [CrossRef]
- Duwiejua, M.; Woode, E.; Obiri, D.D. Pseudo-akuammigine, an alkaloid from Picralima nitida seeds, has anti-inflammatory and analgesic actions in rats. J. Ethnopharmacol. 2002, 81, 73–79. [Google Scholar] [CrossRef]
- Zhou, H.; Mineshita, S. The effect of berberine chloride on experimental colitis in rats in vivo and in vitro. J. Pharm. Exp. Ther. 2000, 294, 822–829. [Google Scholar]
- Yesilada, E.; Kupeli, E. Berberis crataegina DC. root exhibits potent anti-inflammatory, analgesic and febrifuge effects in mice and rats. J. Ethnopharmacol. 2002, 79, 237–248. [Google Scholar] [CrossRef]
- Shen, Y.B.; Piao, X.S; Kim, S.W.; Wang, L.; Liu, P. The effects of berberine on the magnitude of the acute inflammatory response induced by Escherichia coli lipopolysaccharide in broiler chickens. Poult. Sci. 2010, 89, 13–19. [Google Scholar] [CrossRef]
- Das, S.K.; Ramakrishnan, S.; Mishra, K.; Srivastava, R.; Agarwal, G.G.; Singh, R.; Sircar, A.R. A randomized controlled trial to evaluate the slow acting symptom-modifying effects of colchicine in osteoarthritis of the knee: A preliminary report. Arthritis Care Res. 2002, 47, 280–284. [Google Scholar] [CrossRef]
- Idowu, T.O.; Iwalewa, E.O.; Aderogba, M.A.; Akinpelu, B.A.; Ogundaini, A.O. Antinociceptive, anti-inflammatory and antioxidant activities of eleagnine: An alkaloid isolated from Chrysophyllum albidum seed cotyledons. J. Biol. Sci. 2006, 6, 1029–1034. [Google Scholar] [CrossRef]
- Lal, B.; Bhise, N.B.; Gidwani, R.M.; Lakdawala, A.D.; Kalpana, J.; Patvardhan, S. Isolation, synthesis and biological activity of evolitrine and analogs. ARKIVOC 2005, 11, 77–97. [Google Scholar]
- Choi, H.S.; Kim, H.S.; Min, K.R.; Kim, Y.S.; Lim, H.K.; Chang, Y.K.; Chung, M.W. Anti-inflamamtory effects of fangchinoline and tetrandrine. J. Ethnopharmacol. 2000, 69, 173–179. [Google Scholar] [CrossRef]
- Shen, Y.C.; Chou, C.J.; Chiou, W.F.; Chen, C.F. Anti-inflammatory effects of the partially purified extract of radix Stephaniae tetrandrae: Comparative studies of its active principles tetrandrine and fangchinoline on human polymorphonuclear leukocyte functions. Mol. Pharmacol. 2001, 60, 1083–1090. [Google Scholar]
- Manga, H.M.; Haddad, M.; Pieters, L.; Baccelli, C.; Penge, A.; Leclercq, J.Q. Anti-inflammatory compounds from leaves and root bark of Alchornea cordifolia (Schumach. & Thonn.) Müll. Arg. J. Ethnopharmacol. 2008, 115, 25–29. [Google Scholar] [CrossRef]
- Chen, J.J.; Luo, Y.T.; Hwang, T.L.; Sung, P.J.; Wang, T.C.; Chen, I.S. A new indole alkaloid and anti-inflammatory constituents from Strychnos cathayensis. Chem. Biodivers. 2008, 5, 1345–1352. [Google Scholar] [CrossRef]
- Ho, Y.L.; Tsai, H.Y.; Chang, Y.S. Studies on the antinociceptive and antiinflammatory effects of indigo and indirubin in mice. J. Chin. Med. Sci. 2001, 2, 263–271. [Google Scholar]
- Yousaf, M.; Hammond, N.L.; Peng, J.N.; Wahyuono, S.; McIntosh, K.A.; Charman, W.N.; Mayer, A.M.S.; Hamann, M.T. New manzamine alkaloids from an indo-pacific sponge. pharmacokinetics, oral availability, and the significant activity of several manzamines against HIV-I, AIDS opportunistic infections, and inflammatory diseases. J. Med. Chem. 2004, 47, 157–160. [Google Scholar]
- Peng, Z.; Zhang, Z.X.; Xu, Y.J. Effect of ligustrazine on CD11c and CD14 content on alveolar macrophages from patients with chronic bronchitis. Zhongguo Yaolixue Yu Dulixue Zazhi 2000, 14, 157–160. [Google Scholar]
- Fan, Z.; Liu, Y. Anti-inflammatory effects of tetramethylpyrazine. Shenyang Yaoke Daxue Xuebao 2000, 17, 289–291. [Google Scholar]
- Luo, Y.; Liu, M.; Xia, Y.; Dai, Y.; Chou, G.; Wang, Z. Therapeutic effect of norisoboldine, an alkaloid isolated from Radix Linderae, on collagen-induced arthritis in mice. Phytomedicine 2010, 17, 726–731. [Google Scholar] [CrossRef]
- Rho, J.R.; Jun, C.S.; Ha, Y.A.; Yoo, M.J.; Cui, M.X.; Baek, H.S.; Lim, J.A.; Lee, Y.H.; Chai, K.Y. Isolation and characterization of a new alkaloid from seed of Prunus persica L. and its anti-inflammatory activity. Bull. Korean Chem. Soc. 2007, 28, 1289–1293. [Google Scholar] [CrossRef]
- Shang, J.H.; Cai, X.H.; Feng, T.; Zhao, Y.L.; Wang, J.K.; Zhang, L.Y.; Yan, M.; Luo, X.D. Pharmacological evaluation of Alstonia scholaris: Anti-inflammatory and analgesic effects. J. Ethnopharmacol. 2010, 129, 174–181. [Google Scholar] [CrossRef]
- Daware, M.B.; Mujumdar, A.M.; Ghaskabdi, S. Reproductive toxicity of piperine in swiss albino mice. Planta Med. 2000, 66, 231–236. [Google Scholar] [CrossRef]
- Williamson, L.; Illingworth, H.; Smith, D.; Mowat, A. Oral quinine in ankylosing spondylitis: A randomized placebo controlled double blind crossover trial. J. Rheumatol. 2000, 27, 2054–2055. [Google Scholar]
- Araújo, F.L.O.; Melo, C.T.V.; Rocha, N.F.M.; Moura, B.A.; Leite, C.P.; Amaral, J.F.; Barbosa-Filho, J.M.; Gutierrez, S.J.C.; Vasconcelos, S.M.M.; Viana, G.S.B.; et al. Antinociceptive effects of (O-methyl)-N-benzoyl tyramine (riparin I) from Aniba riparia (Nees) Mez (Lauraceae) in mice. Naunyn Schmiedebergs Arch. Pharmacol. 2009, 380, 337–344. [Google Scholar] [CrossRef]
- Stevenson, C.S.; Capper, E.A.; Roshak, A.K.; Marquez, B.; Grace, K.; Gerwick, W.H.; Jacobs, R.S.; Marshall, L.A. Scytonemin – a marine natural product inhibitor of kinases key in hyperproliferative inflammatory diseases. Inflamm. Res. 2002, 51, 112–114. [Google Scholar] [CrossRef]
- Liu, X.L.; Chen, G.X.; Li, X.J.; Huang, Q.C.; Liu, Q.P.; Chen, J.F. Inhibitive effects of sinomenine on inflammatory synovium in rats with arthritis induced by collagen II and its mechanism. Guangzhou Zhongyiyao Daxue Xuebao 2002, 19, 214–217. [Google Scholar]
- Garcia-Argaez, A.N.; Ramirez Apan, T.O.; Delgado, H.P.; Velazquez, G.; Martinez-Varquez, M. Anti-inflammatory activity of coumarins from Decatropis bicolor on TPA ear mice model. Planta Med. 2000, 66, 279–281. [Google Scholar] [CrossRef]
- Xu, L.J.; Wei, S.C.; Lu, F.E.; Huang, G.; Huang, G.Y.; Ye, W.Y. Comparison of effects of several components derived from Strychnos nuxvomica on experimental arthritis. Tongji Yike Daxue Xuebao 2001, 30, 564–565. [Google Scholar]
- Shaheen, F.; Ahmad, M.; Khan, T.H.; Jalil, S.; Ejaz, A.; Sultankhodjaev, M.N.; Arfan, M.; Choudhary, M.I.; Rahman, A.U. Alkaloids of Aconitum laeve and their anti-inflammatory, antioxidant and tyrosinase inhibition activities. Phytochemistry 2005, 66, 935–940. [Google Scholar]
- Wang, Y.; Yang, X.; Zheng, X.; Li, J.; Ye, C.; Song, X. Theacrine, a purine alkaloid with anti-inflammatory and analgesic activities. Fitoterapia 2010, 81, 627–631. [Google Scholar] [CrossRef]
- Naidoo, D.; Coombes, P.H.; Mulholland, D.A.; Crouch, N.R.; van Den Bergh, A.J.J. N-Substituted acridone alkaloids from Toddaliopsis bremekampii (Rutaceae: Toddialoideae) of South-Central Africa. Phytochemistry 2005, 66, 1724–1728. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Souto, A.L.; Tavares, J.F.; Da Silva, M.S.; Diniz, M.d.F.F.M.; De Athayde-Filho, P.F.; Barbosa Filho, J.M. Anti-Inflammatory Activity of Alkaloids: An Update from 2000 to 2010. Molecules 2011, 16, 8515-8534. https://doi.org/10.3390/molecules16108515
Souto AL, Tavares JF, Da Silva MS, Diniz MdFFM, De Athayde-Filho PF, Barbosa Filho JM. Anti-Inflammatory Activity of Alkaloids: An Update from 2000 to 2010. Molecules. 2011; 16(10):8515-8534. https://doi.org/10.3390/molecules16108515
Chicago/Turabian StyleSouto, Augusto Lopes, Josean Fechine Tavares, Marcelo Sobral Da Silva, Margareth de Fátima Formiga Melo Diniz, Petrônio Filgueiras De Athayde-Filho, and José Maria Barbosa Filho. 2011. "Anti-Inflammatory Activity of Alkaloids: An Update from 2000 to 2010" Molecules 16, no. 10: 8515-8534. https://doi.org/10.3390/molecules16108515





