Synthesis and Analgesic Activity of Some New Pyrazoles and Triazoles Bearing a 6,8-Dibromo-2-methylquinazoline Moiety
Abstract
:1. Introduction
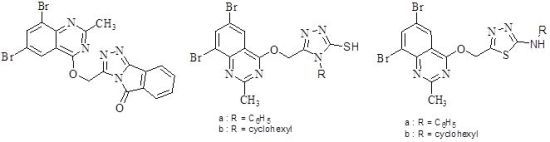
2. Chemistry




3. Pharmacological Studies
3.1. Analgesic Activity
| Comp. NO. | Comparative analgesic potency to Valdecoxib after time in minutes | |||
|---|---|---|---|---|
| 10 min. | 30 min. | 60 min. | 120 min. | |
| 6 | 0.48 ± 0.01 | 0.52 ± 0.04 | 0.68 ± 0.06 | 1.33 ± 0.08 |
| 7 | 0.76 ± 0.03 | 0.98 ± 0.09 | 1.23 ± 0.11 | 2.22 ± 0.20 |
| 9 | 0.44 ± 0.01 | 0.51 ± 0.05 | 0.63 ± 0.06 | 1.29 ± 0.16 |
| 11a | 0.52 ± 0.01 | 0.80 ± 0.07 | 0.87 ± 0.08 | 1.92 ± 0.08 |
| 11c | 0.48 ± 0.02 | 0.59 ± 0.05 | 0.75 ± 0.07 | 1.59 ± 0.04 |
| 13a | 0.65 ± 0.01 | 0.98 ± 0.08 | 1.03 ± 0.01 | 2.25 ± 0.18 |
| 13b | 0.56 ± 0.01 | 0.86 ± 0.05 | 0.98 ± 0.08 | 2.36 ± 0.21 |
| 14a | 0.68 ± 0.02 | 0.88 ± 0.07 | 1.20 ± 0.14 | 2.52 ± 0.14 |
| 14b | 0.60 ± 0.02 | 0.96 ± 0.03 | 1.01 ± 0.01 | 2.35 ± 0.12 |
| Valdecoxib (g) | 1.00 | 1.00 | 1.00 | 1.00 |
3.2. Results
4. Experimental
4.1. General Procedure for the Synthesis of Compounds 10a-c
4.2. General Procedure for the Synthesis of Compounds 11a-c
4.3. General Procedure for the Synthesis of 13a,b
4.4. General Procedure for the Synthesis of 14a,b
5. Conclusions
Acknowledgment
References and Notes
- Pavli, M.; Baumgartner, S.; Kos, P.; Kogej, K. Doxazosin-carrageenan interactions: A novel approach for studying drug-polymer interactions and relation to controlled drug release. Int. J. Pharm. 2011, 421, 110–119. [Google Scholar] [CrossRef]
- Jatav, V.; Mishra, P.; Kashaw, S.; Stables, J.P. CNS depressant and anticonvulsant activities of some novel 3-[5-substituted 1,3,4-thiadiazole-2-yl]-2-styryl quinazoline-4(3H)-ones. Eur. J. Med. Chem. 2008, 43, 1945–1954. [Google Scholar] [CrossRef]
- Hawkins, R.A.; Stephens, C.E. Aerobic epoxidation and hydroxylation of a pyrrolo[2,1-b]quinazoline under ambient conditions. Tetrahedron Lett. 2010, 51, 6129–6131. [Google Scholar] [CrossRef]
- Wakeling, A.E. Discovery and development of Iressa: The first in a new class of drugs targeted at the epidermal growth factor receptor tyrosine kinase. In Inhibitors of Protein Kinases and Protein Phosphatases—Handbook of Experimental Pharmacology; Pinna, L.A., Cohen, P.T.W., Eds.; Springer-Verlag: Berlin, Germany, 2005. [Google Scholar]
- Abdel-Fattah, M.E.; Soliman, E.A.; Soliman, S.M.A. Synthesis and reactions of 2-[2-(2,4,6-trimethylbenzoyl) vinyl]-4H-3,1-benzoxazin-4-one of expected biological activity. Egypt. J. Chem. 1999, 42, 499–516. [Google Scholar]
- Mohan, R.R.; Agarwal, R.; Misra, V.S. Synthesis of some newer quinazolinyl-oxadiazoles, thiosemicarbazides and thiadiazoles as pharmacologically active agents. Indian J. Chem. 1985, 24B, 78–82. [Google Scholar]
- Hernandez, F.; Morales, V.; Buenadicha, F.L.; Soellhuber, M.; Avendano, C. Influence of N(2)-substitution in the alkylation of (4S)-alkyl-2,4-dihydro-1H-pyrazino[2,1-b]quinazoline-3,6-diones. Tetrahedron Asymmetry 2004, 15, 3045–3058. [Google Scholar] [CrossRef]
- Issac, Y.A.; Arsanious, M.H.; Abd El-Nabi, H.A. A facile synthesis of 2-phenylquinazoline derivatives. Egypt. J. Chem. 2002, 45, 929–946. [Google Scholar]
- Nassar, S.A.; Aly, A.A. Synthesis of some new substituted β-(Quinazolin-2-yl) acrylic acid derivatives of expected biological activity. Egypt. J. Chem. 2002, 45, 205–217. [Google Scholar]
- El-Hashash, M.A.; Abdel-Rahman, T.M.; El-Badry, Y.A. Synthesis and behavior of 2-carboxyvinyl-6,8-dibromo-4H-3,1-benzoxazin-4-one towards nitrogen, carbon and sulfur nucleophiles. Ind. J. Chem. Sect. B Org. Chem. Incl. Med. Chem. 2006, 45, 1470–1477. [Google Scholar]
- Ouyang, G.; Zhang, P.; Xu, G.; Song, B.; Yang, S.; Jin, L.; Xue, W.; Hu, D.; Lu, P.; Chen, Z. Synthesis and antifungal bioactivities of 3-alkylquinazolin-4-one derivatives. Molecules 2006, 11, 383–392. [Google Scholar] [CrossRef]
- Chandrika, P.M.; Yakaiah, T.; Rao, A.R.; Narsaiah, B.; Reddy, N.C.; Sridhar, V.; Rao, J.V. Synthesis of novel 4,6-disubstituted quinazoline derivatives, their anti-inflammatory and anti-cancer activity (cytotoxic) against U937 leukemia cell lines. Eur. J. Med. Chem. 2008, 43, 846–852. [Google Scholar] [CrossRef]
- Giri, R.S.; Thaker, H.M.; Giordano, T.; Williams, J.; Rogers, D.; Sudersanam, V.; Vasu, K.K. Design, synthesis and characterization of novel 2-(2,4-disubstituted-thiazole-5-yl)-3-aryl-3H-quinazoline-4-one derivatives as inhibitors of NF-κB and AP-1 mediated transcription activation and as potential anti-inflammatory agents. Eur. J. Med. Chem. 2009, 44, 2184–2189. [Google Scholar] [CrossRef]
- Alafeefy, A.M.; Kadi, A.A.; Al-Deeb, O.A.; El-Tahir, K.E.H.; Al-jaber, N.A. Synthesis, analgesic and anti-inflammatory evaluation of some novel quinazoline derivatives. Eur. J. Med. Chem. 2010, 45, 4947–4952. [Google Scholar] [CrossRef]
- Park, H.J.; Kim, Y.S.; Kim, J.S.; Lee, E.J.; Yi, Y.J.; Hwang, H.J.; Suh, M.E.; Ryu, C.K.; Lee, S.K. 6-Arylamino-7-chloro-quinazoline-5,8-diones as novel cytotoxic and DNA topoisomerase inhibitory agents. Bioorg. Med. Chem. Lett. 2004, 14, 3385–3388. [Google Scholar] [CrossRef]
- Jin, Y.; Zhou, Z.Y.; Tian, W.; Yu, Q.; Long, Y.Q. 4′-Alkoxyl substitution enhancing the anti-mitotic effect of 5-(3′,4′,5′-substituted)anilino-4-hydroxy-8-nitroquinazolines as a novel class of anti-microtubule agents. Bioorg. Med. Chem. Lett. 2006, 16, 5864–5869. [Google Scholar] [CrossRef]
- Kundu, S.K.; Mahindaratne, M.P.D.; Quintero, M.V.; Bao, A.; Negrete, G.R. One-pot reductive cyclization to antitumor quinazoline precursors. ARKIVOC 2008, ii, 33–42. [Google Scholar]
- El-Azab, A.S.; Al-Omar, M.A.; Abdel-Aziz, A.A.M.; Abdel-Aziz, N.I.; El-Sayed, M.A.A.; Aleisa, A.M.; Sayed-Ahmed, M.M.; Abdel-Hamid, S.G. Design, synthesis and biological evaluation of novel quinazoline derivatives as potential antitumor agents: Molecular docking study. Eur. J. Med. Chem. 2010, 45, 4188–4198. [Google Scholar]
- Alagarsamy, V.; Murugesan, S.; Dhanabal, K. Anti-HIV, antibacterial and antifungal activities of some novel 2-methyl-3-(substituted methylamino)-(3H)-quinazolin-4-ones. Indian J. Pharm. Sci. 2007, 69, 304–307. [Google Scholar] [CrossRef]
- Jessy, E.M.; Thirugnana, A.; Alex, J. Synthesis and biological evaluation of some novel quinazolones. Indian J. Pharm. Sci. 2007, 69, 476–478. [Google Scholar] [CrossRef]
- Mohamed, M.S.; Kamel, M.M.; Kassem, E.M.M.; Abotaleb, N.; Abd El-moez, S.I.; Ahmed, M.F. Novel 6,8-dibromo-4(3H)quinazolinone derivatives of anti-bacterial and anti-fungal activities. Eur. J. Med. Chem. 2010, 45, 3311–3319. [Google Scholar] [CrossRef]
- Patel, N.B.; Barat, G.G. In vitro microbial studies of new pyrazolyl quinazolin-4(3H) ones. J. Saudi Chem. Soc. 2010, 14, 157–164. [Google Scholar] [CrossRef]
- Georgey, H.; Abdel-Gawad, N.; Abbas, S. Synthesis and anticonvulsant activity of some quinazolin-4-(3H)-one derivatives. Molecules 2008, 13, 2557–2569. [Google Scholar] [CrossRef]
- Kashaw, S.K.; Kashaw, V.; Mishra, P.; Jain, N.K.; Stables, J.P. Synthesis, anticonvulsant and CNS depressant activity of some new bioactive 1-(4-substituted-phenyl)-3-(4-oxo-2-phenyl/ethyl-4H-quinazolin-3-yl)-urea. Eur. J. Med. Chem. 2009, 44, 4335–4343. [Google Scholar] [CrossRef]
- Marzro, G.; Guiotto, A.; Pastorini, G.; Chilin, A. A novel approach to quinazolin-4(3H)-one via quinazoline oxidation: An improved synthesis of 4-anilinoquinazolines. Tetrahedron 2010, 66, 962–968. [Google Scholar] [CrossRef]
- Gujral, M.L.; Saxena, P.N.; Tiwari, R.S. Comparative evaluation of quinazolones: A new class of hypnotics. Indian J. Med. Res. 1955, 43, 637–641. [Google Scholar]
- Heredia, M.L.; de la Cuesta, E.; Avendano, C. Acid-promoted reactions in 1-hydroxy, 1-dimethylaminomethyl and 1-methylene-4-arylmethyl-2,4-dihydro-1H-pyrazino[2,1-b]quinazoline-3,6-diones. Tetrahedron 2002, 58, 6163–6170. [Google Scholar] [CrossRef]
- Buenadicha, F.L.; Avendaño, C.; Söllhuber, M. Asymmetrically induced alkylation of 2-benzyl-4-isopropyl-2,4-dihydro-1H-pyrazino[2,1-b]quinazoline-3,6-dione. Tetrahedron: Asymmetry 1998, 9, 4275–4284. [Google Scholar] [CrossRef]
- Jones, T.R. 5-Substituted quinazolineantifolates. Eur. J. Cancer 1980, 16, 707–711. [Google Scholar] [CrossRef]
- Heredia, M.L.; Fernández, M.; de la Cuesta, E.; Avendao, C. 1-Bromo-2,4-dihydro-1H-pyrazino[2,1-b]quinazoline-3,6-diones as α-bromoglycine templates. Tetrahedron: Asymmetry 2001, 12, 411–418. [Google Scholar] [CrossRef]
- Lenka, K.; Martin, S.; Katarina, K.; Vladimir, C.; Jitka, V.; LuděK, J.; Pia, V.; Miloš, M.; Jarmila, K. Synthesis and Biological Evaluation of Quinazoline-4-thiones. Molecules 2003, 8, 756–769. [Google Scholar] [CrossRef]
- Zheng, L.W.; Wu, L.L.; Zhao, B.X.; Dong, W.L.; Miao, J.Y. Synthesis of novel substituted pyrazole-5-carbohydrazide hydrazone derivatives and discovery of a potent apoptosis inducer in A549 lung cancer cells. Bioorg. Med. Chem. 2009, 17, 1957–1962. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Liu, L.; Guo, J.; Wu, D.; Xu, G.; Wang, X.; Jia, D. Anticancer activity, structure, and theoretical calculation of N-(1-phenyl-3-methyl-4-propyl-pyrazolone-5)-salicylidene hydrazone and its copper(II) complex. Inorg. Chim. Acta 2010, 363, 289–293. [Google Scholar]
- Rzeski, W.; Matysiak, J.; Szerszen, M.K. Anticancer, neuroprotective activities and computational studies of 2-amino-1,3,4-thiadiazole based compound. Bioorg. Med. Chem. 2007, 15, 3201–3207. [Google Scholar] [CrossRef]
- Al-Soud, Y.A.; Al-Masoudi, N.A.; Abd El-Rahman, S.F. Synthesis and properties of new substituted 1,2,4-triazoles: Potential antitumor agents. Bioorg. Med. Chem. 2003, 11, 1701–1708. [Google Scholar] [CrossRef]
- Moustafa, A.H.; Haggam, R.A.; Younes, M.E.; El Ashry, E.S.H. The Synthesis of Triazolothiadiazines and Thiadiazoles From 1,2-Bis-(4-amino-5-mercapto-1,2,4-triazol-3-yl)Ethanol and Ethane. Phosphorus Sulfur Silicon 2006, 181, 2361–2371. [Google Scholar] [CrossRef]
- Yassin, F.A.; Saad, H. Synthesis and reactions of 2-[2-(3-methyl-4-methoxyhydroxyimino-benzyl)phenyl]-4(3H)-Quinazolinone with carbon and sulphur electrophiles. Chim. Acta Turc. 2000, 28, 15–19. [Google Scholar]
- Zou, X.; Jin, G. Synthesis of pyridazinone-substituted 1,3,4-thiadiazoles, -1,3,4-oxadiazoles and -1,2,4-triazoles. J. Heterocycl. Chem. 2001, 38, 993–996. [Google Scholar] [CrossRef]
- Tjolsen, A.; Rosland, J.H.; Berge, O.G.; Hole, K. The increasing-temperature hot-plate test: An improved test of nociception in mice and rats. J. Pharmacol. Methods 1991, 25, 241–250. [Google Scholar] [CrossRef]
- Mekonnena, T.; Urgab, K.; Engidawork, E. Evaluation of the diuretic and analgesic activities of the rhizomes of RumexabyssinicusJacq in mice. J. Ethnopharmacol. 2010, 127, 433–439. [Google Scholar] [CrossRef]
- Alam, M.M.; Husain, A.; Suruchi, S.M.H.; Anwer, T. Synthesis and pharmacological evaluation of 2(3H)-furanones and 2(3H)-pyrrolones, combining analgesic and anti-inflammatory properties with reduced gastrointestinal toxicity and lipid peroxidation. Eur. J. Med. Chem. 2009, 44, 2636–2642. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the all compounds are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Saad, H.A.; Osman, N.A.; Moustafa, A.H. Synthesis and Analgesic Activity of Some New Pyrazoles and Triazoles Bearing a 6,8-Dibromo-2-methylquinazoline Moiety. Molecules 2011, 16, 10187-10201. https://doi.org/10.3390/molecules161210187
Saad HA, Osman NA, Moustafa AH. Synthesis and Analgesic Activity of Some New Pyrazoles and Triazoles Bearing a 6,8-Dibromo-2-methylquinazoline Moiety. Molecules. 2011; 16(12):10187-10201. https://doi.org/10.3390/molecules161210187
Chicago/Turabian StyleSaad, Hosam A., Nermen A. Osman, and Ahmed H. Moustafa. 2011. "Synthesis and Analgesic Activity of Some New Pyrazoles and Triazoles Bearing a 6,8-Dibromo-2-methylquinazoline Moiety" Molecules 16, no. 12: 10187-10201. https://doi.org/10.3390/molecules161210187