‘Heat-Treatment Aqueous Two Phase System’ for Purification of Serine Protease from Kesinai (Streblus asper) Leaves
Abstract
:1. Introduction
2. Results and Discussions
2.1. Effect of Temperature on Purity of Serine Protease from Kesinai Leaves


2.2. SDS-PAGE Analysis of the Purified Serine Protease
2.3. Effect of PEG on the Partitioning of Serine Protease from Kesinai Leaves
| Phase Composition (%, w/w) | KP | KE | PF | Yield (%) |
|---|---|---|---|---|
| 8% PEG4000-15% MgSO4 | ns | ns | ns | ns |
| 16% PEG4000-15% MgSO4 | 0.24 ± 0.03 | 1.4 ± 0.09 | 4.4 ± 0.13 | 41.3 ± 0.22 |
| 21% PEG4000-15% MgSO4 | 0.50 ± 0.10 | 0.03 ± 0.01 | 3.2 ± 0.10 | 33.8 ± 0.31 |
| 8% PEG6000-15% MgSO4 | 0.22 ± 0.74 | 2.12 ± 0.02 | 6.2 ± 0.8 | 53.4 ± 0.13 |
| 16% PEG6000-15% MgSO4 | 0.001 ± 0.23 | 4.52 ± 0.04 | 8.9 ± 0.02 | 83.1 ± 0.08 |
| 21% PEG6000-15% MgSO4 | 0.04 ± 0.18 | 3.51 ± 0.14 | 7.8 ± 0.2 | 61 ± 0.06 |
| 8% PEG8000-15% MgSO4 | 0.31 ± 0.15 | 1.02 ± 0.12 | 5.3 ± 0.03 | 50.2 ± 0.12 |
| 16% PEG8000-15% MgSO4 | 0.07 ± 0.07 | 0.23 ± 0.01 | 3.9 ± 0.01 | 31.9 ± 0.03 |
| 21% PEG8000-15% MgSO4 | 0.10 ± 0.02 | 0.31 ± 0.21 | 4.2 ± 0.05 | 40.2 ± 0.05 |
2.4. Effect of Salts on the Partitioning of Serine Protease from Kesinai Leaves
| Phase Composition (%,w/w) | KP | KE | PF | Yield (%) |
|---|---|---|---|---|
| 16% PEG6000-12% Na-citrate | ns | ns | ns | ns |
| 16% PEG6000-15% Na-citrate | 0.12 ± 0.02 | 1.8 ± 0.15 | 3.8 ± 0.80 | 34 ± 0.09 |
| 16% PEG6000-18% Na-citrate | 0.24 ± 0.17 | 0.8 ± 0.40 | 1.6 ± 0.17 | 21 ± 0.07 |
| 16% PEG6000-12% MgSO4 | 0.12 ± 0.23 | 4.1 ± 0.31 | 7.4 ± 0.14 | 74 ± 0.07 |
| 16% PEG6000-15% MgSO4 | 0.02 ± 0.01 | 5.38 ± 0.06 | 11.3 ± 0.21 | 87.2 ± 0.4 |
| 16% PEG6000-18% MgSO4 | 0.08 ± 0.11 | 3.4 ± 0.21 | 18.5 ± 0.31 | 62 ± 0.82 |
| 16% PEG6000-12% K2HPO4 | 0.31 ± 0.02 | 2.9 ± 0.21 | 5.1 ± 0.04 | 52 ± 0.10 |
| 16% PEG6000-15% K2HPO4 | 0.23 ± 0.18 | 3.8 ± 0.32 | 6.8 ± 0.22 | 63 ± 0.02 |
| 16% PEG6000-18% K2HPO4 | 0.18 ± 0.04 | 3.1 ± 0.11 | 4.3 ± 0.02 | 48 ± 0.12 |
2.5. Effect of pH on the Partitioning of Serine Protease from Kesinai Leaves
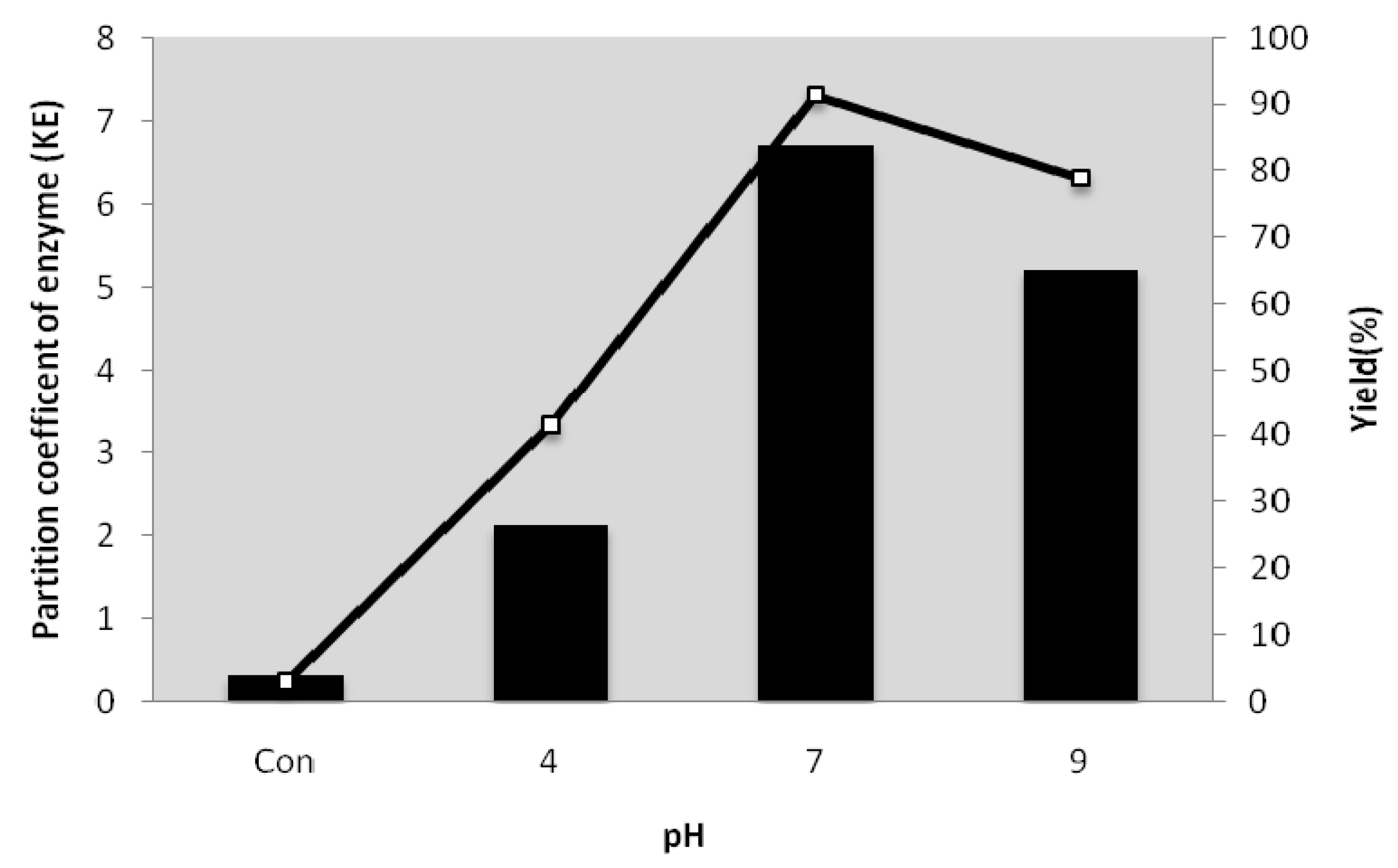
2.6. Effect of NaCl on the Partitioning of Serine Protease from Kesinai Leaves

3. Experimental
3.1. Plant Materials
3.2. Chemicals
3.3. Enzyme Extraction
3.4. Optimization of Heat-Treatment on Crude Enzyme
3.5. Preparation of ‘Heat Treatment Aqueous Two Phase System’
3.6. Effect of PEG on the Partitioning of Serine Protease
3.7. Effects of pH and NaCl on Partitioning of Serine Protease
3.8. Analytical Methods
3.8.1. Serine Protease Activity Assay
3.8.2. Protein Concentration Determination
3.8.3. Determination of Partition Coefficient, Specific Activity, Purification Factor and Yield
3.8.4. Sodium Dodecyl Sulfate-polyacrylamide Gel Electrophoresis (SDS-PAGE)
4. Conclusions
References and Notes
- Aehle, W. Enzymes in Industry: Production and Application; Wiley: New York, NY, USA, 2004. [Google Scholar]
- Antão, C.M.; Malcata, F.X. Plant serine proteases: Biochemical, physiological and molecular features. Plant Physiol. Biochem. 2005, 43, 637–650. [Google Scholar] [CrossRef]
- Asif-Ullah, M.; Kim, K.; Yu, Y.G. Purification and characterization of a serine protease from Cucumis trigonus Roxburghi. Photochemistry 2006, 67, 870–875. [Google Scholar] [CrossRef]
- Rastogi, S.; Kulshreshtha, D.K.; Rawat, A.K.S. Streblus asper Lour. (Shakhotaka): A review of its chemical, pharmacological and ethnomedicinal propertie. eCAM 2006, 3, 217–222. [Google Scholar]
- Taweechaisupapong, S.; Choopan, T.; Singhara, S.; Chatrchaiwiwatana, S.; Wongkham, S. In vitro inhibitory effect of Streblus asper leaf-extract on adhesion of Candida albicans to human buccal epithelial cells. J. Ethnopharmacol. 2005, 96, 221–226. [Google Scholar] [CrossRef]
- Amid, M.; Shuhaimi, M.; Zaidul, I.S.; Yazid, A.M. Optimization of the conditions for extraction of serine protease from Kesinai plant (Streblus asper) leaves using response surface methodology. Molecules 2011, 16, 8419–8427. [Google Scholar] [CrossRef]
- Zhang, M.; Hub, P.; Liang, Q.; Yang, H.; Liu, Q.; Wang, Y.; Luoa, G. Direct process integration of extraction and expanded bed adsorption in the recovery of crocetin derivatives from Fructus gardenia. J. Chromatogr. B 2007, 858, 220–226. [Google Scholar] [CrossRef]
- Hatti-Kaul, R. Aqueous Two-Phase Systems: Methods and Protocols; Humana Press: Totawa, NJ, USA, 2001. [Google Scholar]
- Chaiwut, P.; Rawdkuen, S.; Benjakul, S. Extraction of protease from Calotropis procera latex by polyethylene glycol-salts biphasic system. Process Biochem. 2010, 45, 680–685. [Google Scholar]
- Bim, M.A.; Franco, T.T. Extraction in aqueous two-phase systems of alkaline xylanase produced by Bacillus pumillus and its application in kraff pulp bleaching, J. Chromatogr. B 2000, 743, 349–356. [Google Scholar] [CrossRef]
- Babu, B.; Rastogi, N.K.; Raghavarao, M.S. Liquid-liquid extraction of bromelain and polyphenol oxidase using aqueous two-phase system. Chem. Eng. Process. 2008, 47, 83–89. [Google Scholar] [CrossRef]
- Wang, W.; Wan, J.; Ning, B.; Xia, J.; Cao, X. Preparation of a novel light-sensitive copolymer and its application in recycling aqueous two-phase systems. J. Chromatogr A 2008, 1205, 171–176. [Google Scholar]
- Lima, Á.S.; Alegre, R.M.; Meirelles, A.J.A. Partitioning of pectinolytic enzymes in polyethylene glycol/potassium phosphate aqueous two-phase systems. Carbohyd. Polym. 2005, 50, 63–68. [Google Scholar]
- Huddleston, J.; Veide, A.; Kohlez, K.; Flanagan, J.; Enfors, S.O.; Lyddiatt, A. The molecular basis of partitioning in ATPS: Review. Trends Biotechnol. 1991, 9, 381–388. [Google Scholar] [CrossRef]
- Roe, S. Protein Purification Techniques: A Practical Approach; Oxford University Press: Oxford, UK, 2000. [Google Scholar]
- Rahimpour, F.; Feyzi, F.; Machsoudi, S.; Hatti-Kaulr, R. Purification of plasmid DNA with polymr-salt aqueous two-phase system: Optimization using response surface methodology. Biotechnol. Bioeng. 2006, 95, 627–637. [Google Scholar] [CrossRef]
- Garcia-Carreno, F.C.; Dimes, C.E.; Haard, N.F. Substrate gel electrophoresis for composition and molecular weight of proteinases or proteinaceous proteinase inhibitors. Anal. Biochem. 1993, 214, 65–69. [Google Scholar]
- Asenjo, J.A.; Schmidt, A.S.; Hachem, F.; Andrew, B.A. Model for predicting the partition behaviour of proteins in aqueous two-phase systems. J. Chromatogr. A 1994, 668, 47–54. [Google Scholar] [CrossRef]
- Albertsson, P.A. Partition of Cell Particles and Macromolecules; Wiley: New York, NY, USA, 1987. [Google Scholar]
- Abbott, N.L.; Hatton, T.A. Liquid-liquid extraction for protein separations. Chem. Eng. Prog. 1988, 84, 31–41. [Google Scholar]
- Marcos, J.C.; Fonseca, L.P.; Ramalho, M.T.; Cabral, J.M.S. Partial purification of penicillin acylase from Escherichia coli in poly (ethylene glycol)-sodium citrate aqueous two-phase systems. J. Chromatogr. B 1999, 734, 15–22. [Google Scholar] [CrossRef]
- Whooley, M.A.; O’Callaghan, J.A.; McLoughlin, A.J. Effect of substrate on the regulation of exprotease production by Pseudomonas aeruginosa ATCC10145. J. Gen. Microbiol. 1983, 129, 981–988. [Google Scholar]
- Costa, M.J.L.; Cunha, M.T.; Cabral, J.M.S.; Aires-Barros, M.R. Scale-up of recombinant cutinase recovery by whole broth extraction with PEG-phosphate aqueous two-phase. Bioseparation 2000, 9, 231–238. [Google Scholar] [CrossRef]
- Mirjana, G.A. Partitioning of pectinase produced by Polyporus squamosus in aqueous two-phase system polyethylene glycol 4000/crude dextran at different initial pH values. Carbohydr. Polym. 2004, 56, 295–300. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Blum, H.; Beier, H.; Gross, H.J. Improved silver staining of plant-proteins, RNA and DNA in polyacrylamide gels. Electrophoresis 1987, 8, 93–99. [Google Scholar] [CrossRef]
- Samples Availability: Not available.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mehrnoush, A.; Mustafa, S.; Yazid, A.M.M. ‘Heat-Treatment Aqueous Two Phase System’ for Purification of Serine Protease from Kesinai (Streblus asper) Leaves. Molecules 2011, 16, 10202-10213. https://doi.org/10.3390/molecules161210202
Mehrnoush A, Mustafa S, Yazid AMM. ‘Heat-Treatment Aqueous Two Phase System’ for Purification of Serine Protease from Kesinai (Streblus asper) Leaves. Molecules. 2011; 16(12):10202-10213. https://doi.org/10.3390/molecules161210202
Chicago/Turabian StyleMehrnoush, Amid, Shuhaimi Mustafa, and Abdul Manap Mohd Yazid. 2011. "‘Heat-Treatment Aqueous Two Phase System’ for Purification of Serine Protease from Kesinai (Streblus asper) Leaves" Molecules 16, no. 12: 10202-10213. https://doi.org/10.3390/molecules161210202




