Use of Titanium Dioxide Photocatalysis on the Remediation of Model Textile Wastewaters Containing Azo Dyes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of TiO2 Photocatalyst Concentration
 ) and C.I Reactive Black 5 (
) and C.I Reactive Black 5 (  ) dyes in 120 min of irradiation with2,60 mW/cm2 of irradiation power by a 125 W mercury lamp.
) dyes in 120 min of irradiation with2,60 mW/cm2 of irradiation power by a 125 W mercury lamp.
 ) and C.I Reactive Black 5 (
) and C.I Reactive Black 5 (  ) dyes in 120 min of irradiation with2,60 mW/cm2 of irradiation power by a 125 W mercury lamp.
) dyes in 120 min of irradiation with2,60 mW/cm2 of irradiation power by a 125 W mercury lamp.
2.2. Effect of UV-Irradiation Time
 ) and C.I Reactive Black 5 (
) and C.I Reactive Black 5 (  ) with 0.1 g·L−1 of TiO2 and 2.60 mW/cm2 of irradiation power by a 125 W mercury lamp.
) with 0.1 g·L−1 of TiO2 and 2.60 mW/cm2 of irradiation power by a 125 W mercury lamp.
 ) and C.I Reactive Black 5 (
) and C.I Reactive Black 5 (  ) with 0.1 g·L−1 of TiO2 and 2.60 mW/cm2 of irradiation power by a 125 W mercury lamp.
) with 0.1 g·L−1 of TiO2 and 2.60 mW/cm2 of irradiation power by a 125 W mercury lamp. 
 ) and C.I Reactive Black 5 (
) and C.I Reactive Black 5 (  ) with 1 g·L−1 of TiO2 and 2.60 mW/cm2 of irradiation power by a 125 W mercury lamp.
) with 1 g·L−1 of TiO2 and 2.60 mW/cm2 of irradiation power by a 125 W mercury lamp.
 ) and C.I Reactive Black 5 (
) and C.I Reactive Black 5 (  ) with 1 g·L−1 of TiO2 and 2.60 mW/cm2 of irradiation power by a 125 W mercury lamp.
) with 1 g·L−1 of TiO2 and 2.60 mW/cm2 of irradiation power by a 125 W mercury lamp.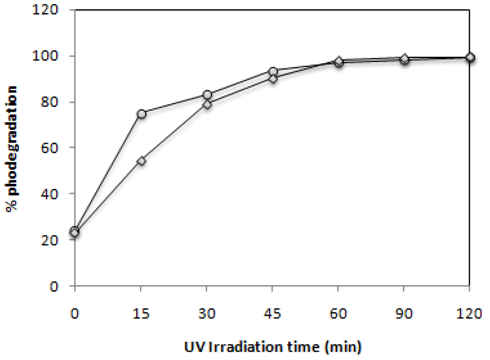
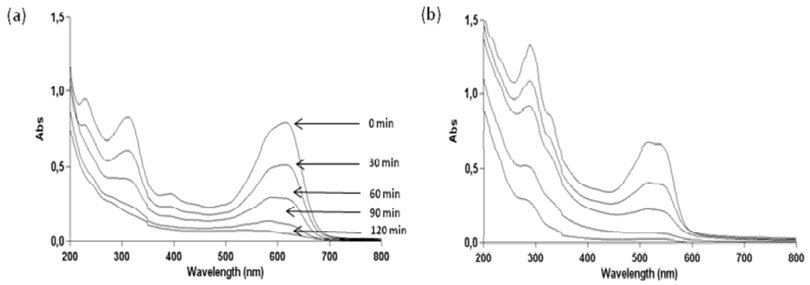
2.3. Effect of pH

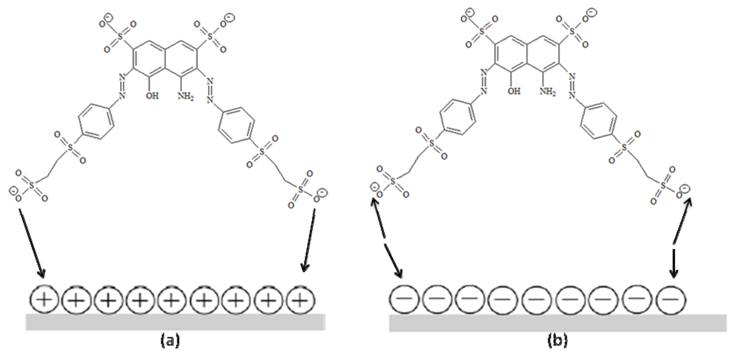
2.4. Recycling of TiO2

2.5. Effect of Dye Concentration
 ) and C.I Reactive Black 5 (
) and C.I Reactive Black 5 (  ) dyes on the efficiency attained after 2 h of irradiation with 0.1 g·L−1 of TiO2 and 2,60 mW/cm2 of irradiation power by a 125 W mercury lamp.
) dyes on the efficiency attained after 2 h of irradiation with 0.1 g·L−1 of TiO2 and 2,60 mW/cm2 of irradiation power by a 125 W mercury lamp.
 ) and C.I Reactive Black 5 (
) and C.I Reactive Black 5 (  ) dyes on the efficiency attained after 2 h of irradiation with 0.1 g·L−1 of TiO2 and 2,60 mW/cm2 of irradiation power by a 125 W mercury lamp.
) dyes on the efficiency attained after 2 h of irradiation with 0.1 g·L−1 of TiO2 and 2,60 mW/cm2 of irradiation power by a 125 W mercury lamp. 
2.6. Effect of H2O2
 ) and C.I Reactive Black 5 (
) and C.I Reactive Black 5 (  ) dyes after 60 min of irradiationwith 0.1g−L−1 of TiO2 and 2,60 mW/cm2 of irradiation power by a 125 W mercury lamp.
) dyes after 60 min of irradiationwith 0.1g−L−1 of TiO2 and 2,60 mW/cm2 of irradiation power by a 125 W mercury lamp.
 ) and C.I Reactive Black 5 (
) and C.I Reactive Black 5 (  ) dyes after 60 min of irradiationwith 0.1g−L−1 of TiO2 and 2,60 mW/cm2 of irradiation power by a 125 W mercury lamp.
) dyes after 60 min of irradiationwith 0.1g−L−1 of TiO2 and 2,60 mW/cm2 of irradiation power by a 125 W mercury lamp. 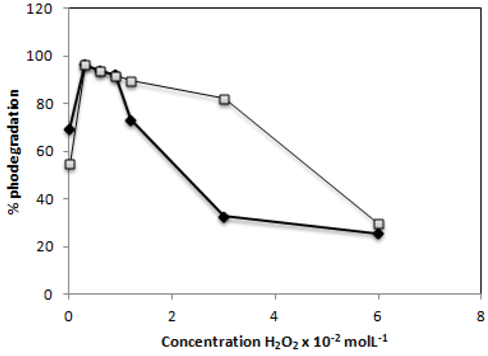
2.7. Effect of the Mixture of the Two Azo Dyes

 ) and C.I Reactive Black 5 (
) and C.I Reactive Black 5 (  ) with (a) 0.1 g·L−1 and (b) 1 g·L−1 of TiO2 and 2.60 mW/cm2 of irradiation power by a 125 W mercury lamp.
) with (a) 0.1 g·L−1 and (b) 1 g·L−1 of TiO2 and 2.60 mW/cm2 of irradiation power by a 125 W mercury lamp.
 ) and C.I Reactive Black 5 (
) and C.I Reactive Black 5 (  ) with (a) 0.1 g·L−1 and (b) 1 g·L−1 of TiO2 and 2.60 mW/cm2 of irradiation power by a 125 W mercury lamp.
) with (a) 0.1 g·L−1 and (b) 1 g·L−1 of TiO2 and 2.60 mW/cm2 of irradiation power by a 125 W mercury lamp. 
3. Experimental
3.1. Reagents and Materials

3.2. Experiments Procedure
3.3. Effect of TiO2 Photocatalyst Concentration
3.4. Effect of pH
3.5. Recycling of TiO2
3.6. Effect of Dye Concentration
3.7. Effect of H2O2
3.8. Effect of the Mixture of the Two Azo Dyes
3.9. Equipment
4. Conclusions
Acknowledgements
References and Notes
- Chen, C.; Wang, Z.; Ruan, S.; Zou, B.; Zhao, M.; Wu, F. Photocatalytic degradation of C.I. Acid Orange 52 in the presence of Zn-doped TiO2 prepared by a stearic acid gel method. Dyes Pigm. 2008, 77, 204–209. [Google Scholar] [CrossRef]
- Vautier, M.; Guillard, C.; Herrmann, J. Photocatalytic Degradation of Dyes in Water: Case Study of Indigo and of Indigo Carmine. J. Catal. 2001, 201, 46–59. [Google Scholar]
- Wang, C.; Lee, C.; Lyu, M.; Juang, L. Photocatalytic degradation of C.I. Basic Violet 10 using TiO2 catalysts supported by Y zeolite: An investigation of the effects of operational parameters. Dyes Pigm. 2008, 76, 817–824. [Google Scholar] [CrossRef]
- Zainal, Z.; Hui, L.K.; Hussein, M.Z.; Taufiq-Yap, Y.H.; Abdullah, A.H.; Ramli, I. Removal of dyes using immobilized titanium dioxide illuminated by fluorescent lamps. J. Hazard. Mater. 2005, 125, 113–120. [Google Scholar] [CrossRef]
- Behnajady, M.A.; Modirshahla, N.; Daneshvar, N.; Rabbani, M. Photocatalytic degradation of an azo dye in a tubular continuous-flow photoreactor with immobilized TiO2 on glass plates. Chem. Eng. J. 2007, 127, 167–176. [Google Scholar] [CrossRef]
- Robinson, T.; McMullan, G.; Marchant, R.; Nigam, P. Remediation of dyes in textile effluent: A critical review on current treatment technologies with a proposed alternative. Bioresour. Technol. 2001, 77, 247–255. [Google Scholar] [CrossRef]
- Habibi, M.H.; Hassanzadeh, A.; Mahdavi, S. The effect of operational parameters on the photocatalytic degradation of three textile azo dyes in aqueous TiO2 suspensions. J. Photochem. Photobiol. A Chem. 2005, 172, 89–96. [Google Scholar] [CrossRef]
- Bizani, E.; Fytianos, K.; Poulios, I.; Tsiridis, V. Photocatalytic decolorization and degradation of dye solutions and wastewaters in the presence of titanium dioxide. J. Hazard. Mater. 2006, 136, 85–94. [Google Scholar] [CrossRef]
- Chen, C.; Lu, C.; Chung, Y. Photocatalytic degradation of ethyl violet in aqueous solution mediated by TiO2 suspensions. J. Photochem. Photobiol. A Chem. 2006, 181, 120–125. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Saggioro, E.M.; Barbosa, N.R.; Mazzei, A.; Ferreira, L.F.V.; Moreira, J.C. Surface Photocatalysis: A Study of the Thickness of TiO2 Layers on the Photocatalytic Decomposition of Soluble Indigo Blue Dye. Rev. Chim. (Bucharest, Rom.) 2011, 62, 462–468. [Google Scholar]
- Sojić, D.; Despotović, V.; Abramović, B.; Todorova, N.; Giannakopoulou, T.; Trapalis, C. Photocatalytic Degradation of Mecoprop and Clopyralid in Aqueous Suspensions of Nanostructured N-doped TiO2. Molecules 2010, 15, 2994–3009. [Google Scholar] [CrossRef]
- Vasapollo, G.; Mele, G.; Sole, R.D.; Pio, I.; Li, J.; Mazzetto, S.E. Use of Novel Cardanol-Porphyrin Hybrids and Their TiO2-Based Composites for the Photodegradation of 4-Nitrophenol in Water. Molecules 2011, 16, 5769–5784. [Google Scholar] [CrossRef]
- Gouvêa, C.A.K.; Wypych, F.; Moraes, S.G.; Durán, N.; Nagata, N.; Peralta-Zamora, P. Semiconductor-assisted Photocatalytic Degradation of Reactive Dyes in Aqueous Solution. Chemosphere 2000, 40, 433–440. [Google Scholar] [CrossRef]
- Gonçalves, M.S.T.; Campos, A.M.F.O.; Pinto, E.M.M.S.; Plasencia, P.M.S.; Queiroz, M.J.R.P. Photochemical treatment of solutions of azo dyes containing TiO2. Chemosphere 1999, 39, 781–786. [Google Scholar] [CrossRef]
- Tariq, M.A.; Faisal, M.; Saquib, M.; Muneer, M. Heterogeneous photocatalytic degradation of an anthraquinone and a triphenylmethane dye derivative in aqueous suspensions of semiconductor. Dyes Pigm. 2008, 76, 358–365. [Google Scholar] [CrossRef]
- Saien, J.; Soleymani, A.R. Degradation and mineralization of Direct Blue 71 in a circulating upflow reactor by UV/TiO2 process and employing a new method in kinetic study. J. Hazard. Mater. 2007, 144, 506–512. [Google Scholar] [CrossRef]
- Aarthi, T.; Narahari, P.; Madras, G. Photocatalytic degradation of Azure and Sudan dyes using nano TiO2. J. Hazard. Mater. 2007, 149, 725–734. [Google Scholar] [CrossRef]
- Saquib, M.; Abu Tariq, M.; Haque, M.M.; Muneer, M. Photocatalytic degradation of disperse blue 1 using UV/TiO2/H2O2 process. J. Environ. Manage. 2008, 88, 300–306. [Google Scholar] [CrossRef]
- Huang, M.; Xu, C.; Wu, Z.; Huang, Y.; Lin, J.; Wu, J. Photocatalytic discolorization of methyl orange solution by Pt modified TiO2 loaded on natural zeolite. Dyes Pigm. 2008, 77, 327–334. [Google Scholar] [CrossRef]
- Andronic, L.; Duta, A. TiO2 thin films for dyes photodegradation. Thin Solid Films 2007, 515, 6294–6297. [Google Scholar] [CrossRef]
- Liu, H.L.; Chiou, Y.R. Optimal decolorization efficiency of Reactive Red 239 by UV/TiO2 photocatalytic process coupled with response surface methodology. Chem. Eng. J. 2005, 112, 173–179. [Google Scholar] [CrossRef]
- Bergamini, R.B.M.; Azevedo, E.B.; Araújo, L.R.R. Heterogeneous photocatalytic degradation of reactive dyes in aqueous TiO2 suspensions: Decolorization kinetics. Chem. Eng. J. 2009, 149, 215–220. [Google Scholar] [CrossRef]
- Zielinska, B.; Grzechulska, J.; Grzmil, B.; Morawski, A.W. Photocatalytic degradation od Reactive Black 5 A comparison between TiO2-Tytanpol A11 and TiO2-Degussa P25 photocatalysts. Appl. Catal.B 2001, 35, L1–L7. [Google Scholar] [CrossRef]
- Alaton, I.A.; Balcioglu, I.A. Photochemical and Heterogeneous photocatalytic degradation of waste vinylsulphone dyes: A case study with hydrolysed Reactive Black 5. J. Photochem. Photobiol. A Chem. 2001, 141, 247–254. [Google Scholar] [CrossRef]
- Muruganandham, M.; Sobana, N.; Swaminathan, M. Solar assited photocatalytic and photochemical degradation of Reactive Black 5. J. Hazard. Mater. 2006, 137, 1371–1376. [Google Scholar] [CrossRef]
- Tang, C.; Chen, V. The photocatalytic degradation of reactive black 5 using TiO2/UV in an annular photoreactor. Water Res. 2004, 38, 2775–2781. [Google Scholar] [CrossRef]
- Saquib, M.; Munner, M. Photocatalytic degradation of two selected textile dye derivates, Eosine Yellowish and ρ-Rosaniline, aqueous suspensions of titanium dioxide. J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. Eng. 2003, 38, 2581–2598. [Google Scholar] [CrossRef]
- Fox, M.A.; Dulay, M.T. Heterogeneous Photocatalysis. Chem. Rev. 1993, 93, 341–357. [Google Scholar]
- Konstantinou, I.K.; Albanis, T.A. TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: kinetic and mechanistic investigations: A review. Appl. Catal. B 2004, 49, 1–14. [Google Scholar] [CrossRef]
- Feng, W.; Nansheng, D.; Helin, H. Degradation mechanism of azo dye C.I. reactive red 2 by iron powder reduction and photooxidation in aqueous solutions. Chemosphere 2000, 41, 1233–1238. [Google Scholar] [CrossRef]
- Karkmaz, M.; Puzenat, E.; Guillard, C.; Herrmann, J.M. Photocatalytic degradation of the alimentary azo dye amaranth. Mineralization of the azo group to nitrogen. Appl. Catal. B 2004, 51, 183–194. [Google Scholar] [CrossRef]
- Mozia, S.; Tomaszewska, M.; Morawski, A.W. Photodegradation of azo dye Acid Red 18 in a quartz labyrinth flow reactor with immobilized TiO2 bed. Dyes Pigm. 2007, 75, 60–66. [Google Scholar] [CrossRef]
- Al-Ekabi, H.; Serpone, N. Photocatalytic degradation of chlorinated phenols in aerated aqueous-solutions over TiO2 supported on a glass matrix. J. Phys. Chem. 1988, 92, 5726–5731. [Google Scholar] [CrossRef]
- Wang, N.; Li, J.; Zhu, L.; Dong, Y.; Tang, H. Highly photocatalytic activity of metallic hydroxide/titanium dioxide nanoparticules prepared via a modified wet precipitation process. J. Photochem. Photobiol. A Chem. 2008, 198, 282–287. [Google Scholar] [CrossRef]
- Dostanic, J.M.; Loncarevic, D.R.; Bankovic, P.T.; Cvetkovik, O.G.; Jovanovic, D.M.; Mijin, D.Z. Influence of process parameters on the photodegradation of synthesized azo pyridine dye in TiO2 water suspension under simulated sunlight. J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. Eng. 2011, 46, 70–79. [Google Scholar] [CrossRef]
- Xie, Y.; Yuan, C. Transparent TiO2 sol nanocrystallites mediated homogeneous-like photocatalytic reaction and hydrosol recycling process. J. Mater. Sci. 2005, 40, 6375–6383. [Google Scholar] [CrossRef]
- Han, F.; Kambala, V.S.R.; Srinivasan, M. Tailored titanium dioxide photocatalysts for the degradation of organic dyes in wastewater treatment: A review. Appl. Catal. A 2009, 359, 25–40. [Google Scholar] [CrossRef]
- Muruganandham, M.; Swaminathan, M. Photocatalytic decolourisation and degradation of Reactive Orange 4 by TiO2-UV process. Dyes Pigm. 2006, 68, 133–142. [Google Scholar] [CrossRef]
- Daneshvar, N.; Salari, D.; Khataee, A.R. Photocatalytic degradation of azo dye acid red 14 in water: investigation of the effect of operational parameters. J. Photochem. Photobiol. A Chem. 2003, 157, 111–116. [Google Scholar] [CrossRef]
- Grzechulska, J.; Morawski, A.W. Photocatalytic decomposition of azo-dye acid black 1 in water over modified titanium dioxide. Appl. Catal. B 2002, 36, 45–51. [Google Scholar] [CrossRef]
- Carter, S.R.; Stefan, M.I.; Bolton, J.R.; Safarzadeh-Amiri, A. UV/H2O2 Treatment of methyl tert-butyl ether in contaminated waters. Environ. Sci. Technol. 2000, 34, 659–662. [Google Scholar] [CrossRef]
- Malato, S.; Blanco, J.; Ritchter, C.; Braun, B.; Maldonado, M.I. Enhancement of the rate of solar photocatalytic mineralization of organic pollutants by inorganic oxidizing species. Appl. Catal. B 1998, 17, 347–356. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Arami, M.; Limaee, N.Y.; Gharanjig, K.; Ardejani, F.D. Decolorization and mineralization of textile dyes at solution bulk by heterogeneous nanophotocatalysis using immobilized nanoparticles of titanium dioxide. Colloids Surf. A 2006, 290, 125–131. [Google Scholar] [CrossRef]
- Prado, A.G.S.; Bolzon, L.B.; Pedroso, C.P.; Moura, A.O.; Costa, L.L. Nb2O5 as efficient and recyclable photocatalyst for indigo carmine degradation. Appl. Catal. A 2008, 82, 219–224. [Google Scholar] [CrossRef]
- Aguedacha, A.; Brosillonb, S.; Morvanb, J.; Lhadi, E.K. Photocatalytic degradation of azo-dyes reactive black 5 and reactive yellow 145 in water over a newly deposited titanium dioxide. Appl. Catal. B 2005, 57, 55–62. [Google Scholar] [CrossRef]
- Zhu, C.; Wang, L.; Kong, L.; Yang, X.; Wang, L.; Zheng, S.; Chen, F.; Maizhi, F.; Zong, H. Photocatalytic degradation of AZO dyes by supported TiO2/UV in aqueous solution. Chemosphere 2000, 41, 303–309. [Google Scholar] [CrossRef]
- Hachem, C.; Bocquillon, F.; Zahraa, O.; Bouchy, M. Decolourization of textile industry wastewater by the photocatalytic degradation process. Dyes Pigm. 2001, 49, 117–125. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Saggioro, E.M.; Oliveira, A.S.; Pavesi, T.; Maia, C.G.; Ferreira, L.F.V.; Moreira, J.C. Use of Titanium Dioxide Photocatalysis on the Remediation of Model Textile Wastewaters Containing Azo Dyes. Molecules 2011, 16, 10370-10386. https://doi.org/10.3390/molecules161210370
Saggioro EM, Oliveira AS, Pavesi T, Maia CG, Ferreira LFV, Moreira JC. Use of Titanium Dioxide Photocatalysis on the Remediation of Model Textile Wastewaters Containing Azo Dyes. Molecules. 2011; 16(12):10370-10386. https://doi.org/10.3390/molecules161210370
Chicago/Turabian StyleSaggioro, Enrico Mendes, Anabela Sousa Oliveira, Thelma Pavesi, Cátia Gil Maia, Luis Filipe Vieira Ferreira, and Josino Costa Moreira. 2011. "Use of Titanium Dioxide Photocatalysis on the Remediation of Model Textile Wastewaters Containing Azo Dyes" Molecules 16, no. 12: 10370-10386. https://doi.org/10.3390/molecules161210370



