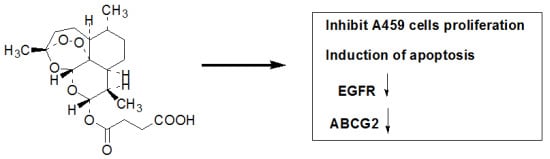
The Effects of Artesunate on the Expression of EGFR and ABCG2 in A549 Human Lung Cancer Cells and a Xenograft Model
Abstract
:1. Introduction
2. Results and Discussion
2.1. ART Inhibits the Growth and Proliferation of the A549 Cell Line

2.2. ART Induces Apoptosis in A549 Cells in a Dose-Dependent Manner
2.3. The Effects of ART on ABCG2 and EGFR mRNA Levels in Lung Cancer Cells

2.4. The Effects of ART on EGFR, Akt and ABCG2 Protein Levels in Lung Cancer Cells
2.5. ART Suppresses Tumor Growth in a Mouse Xenograft Tumor Model

2.6. ART Suppresses EGFR, Akt and ABCG2 Protein and Gene Expression in Vivo

2.7. Discussion
3. Experimental
3.1. Reagents
3.2. Cells and Treatments
3.3. Proliferation Assays
3.4. Apoptosis
3.5. Animal Experiments
3.6. Real-time RT-PCR Analysis
| GenBank | |||
|---|---|---|---|
| Gene | Accession# | Forward | Reverse |
| β-actin | NM-001101.3 | GTGAAGGTGACAGCAGTCGGTT | GAAGTGGGGTGGCTTTTAGGA |
| EGFR | NM-005228.3 | TTTGGGAGTTGATGACCTTTGG | ACGGAACTTTGGGCGACTATCT |
| ABCG2 | NM_031512 | TGAAACCTGGTCTCAACGCCATCC | CGTCAGAGTGCCCATCACAACAT |
3.7. Immunohistochemical Staining
3.8. Western Blotting
3.9. Statistical Analysis
4. Conclusions
Acknowledgements
References and Notes
- Schiller, J.H.; David, H.; Belani, C.P.; Corey, L.; Alan, S.; James, K.; Zhu, J.M.; David, H.J. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N. Engl. J. Med. 2002, 346, 92–98. [Google Scholar] [CrossRef]
- Fukuoka, M.; Yano, S.; Giaccone, G.; Tamura, T.; Nakagawa, K.; Douillard, J.Y.; Nishiwaki, Y.; Vansteenkiste, J.; Kudoh, S.; Rischin, D.; Eek, R. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial) [corrected]. J. Clin. Oncol. 2003, 21, 2237–2246. [Google Scholar] [CrossRef]
- Sequist, L.V.; Bell, D.W.; Lynch, T.J.; Haber, D.A. Molecular predictors of response to epidermal growth factor receptor antagonists in non-small-cell lung cancer. J. Clin. Oncol. 2007, 25, 587–595. [Google Scholar] [CrossRef]
- Lynch, T.J.; Bell, D.W.; Sordella, R.; Gurubhagavatula, S.; Okimoto, R.A.; Brannigan, B.W.; Harris, P.L.; Haserlat, S.M.; Jeffrey, G.; Supko, J.G.; Haluska, F.G.; et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 2004, 350, 2129–2139. [Google Scholar] [CrossRef]
- Paez, J.G.; Janne, P.A.; Lee, J.C.; Tracy, S.; Greulich, H.; Gabriel, S.; Herman, P.; Kaye, F.J.; Lindeman, N.; Boggon, T.J.; et al. EGFR mutations in lung cancer: Correlation with clinical response to gefitinib therapy. Science 2004, 304, 1497–1500. [Google Scholar] [CrossRef]
- Litman, T.; Druley, T.E.; Stein, W.D.; Bates, S.E. From MDR to MXR: New understanding of multidrug resistance systems, their properties and clinical significance. Cell Mol. Life Sci. 2001, 58, 931–959. [Google Scholar] [CrossRef]
- Haimeur, A.; Conseil, G.; Deeley, R.G.; Cole, S.P. The MRP related and BCRP/ABCG2 multidrug resistance proteins: Biology, substrate specificity and regulation. Curr. Drug Metab. 2004, 5, 21–53. [Google Scholar] [CrossRef]
- Sarkadi, B.; Laczka, C.O.; Nemet, K.; Varadi, A. ABCG2—A transporter for all seasons. FEBS Lett. 2004, 567, 116–120. [Google Scholar] [CrossRef]
- Fisher, G.A.; Lum, B.L.; Hausdorff, J.; Sikic, B.I. Pharmacological considerations in the modulation of multidrug resistance. Eur. J. Cancer 1996, 32A, 1082–1088. [Google Scholar]
- Bakos, E.; Evers, R.; Sinko, E.; Radi, A.S.; Borst, P.; Sarkadi, B.Z. Interactions of the human multidrug resistance proteins MRP1 and MRP2 with organic anions. Mol. Pharmacol. 2000, 57, 760–768. [Google Scholar]
- Ross, D.D.; Karp, J.E.; Chen, T.T.; Doyle, L.A. Expression of breast cancer resistance protein in blast cells from patients with acute leukemia. Blood 2000, 96, 365–368. [Google Scholar]
- Brangi, M.; Litman, T.; Ciotti, M.; Nishiyama, K.; Kohlhagen, G.; Takimoto, C.; Robey, R.; Pommier, Y.; Fojo, T.; Bates, S.E. Camptothecin resistance: Role of the ATP-binding cassette (ABC), mitoxantrone-resistance half-transporter (MXR), and potential for glucuronidation in MXR-expressing cells. Cancer Res. 1999, 59, 5938–5946. [Google Scholar]
- Doyle, L.A.; Yang, W.; Abruzzo, L.V.; Krogmann, T.; Gao, Y.M.; Rishi, A.K.; Ross, D.D. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc. Natl. Acad. Sci. USA 1998, 95, 15665–15670. [Google Scholar]
- Allen, J.D.; Schinkel, A.H. Multidrug resistance and pharmacological protection mediated by the breast cancer resistance protein (BCRP/ABCG2). Mol. Cancer Ther. 2002, 1, 427–434. [Google Scholar] [CrossRef]
- Elkind, N.B.; Szentpétery, Z.; Apáti, A.; Laczka, C.O.; Várady, G.; Ujhelly, O.; Szabó, K.; Homolya, L.; Váradi, A.; Buday, L.; et al. Multidrug transporter ABCG2 prevents tumor cell death induced by the epidermal growth factor receptor inhibitor Iressa (ZD1839, Gefitinib). Cancer Res. 2005, 65, 1770–1777. [Google Scholar]
- Haura, E.B.; Tanvetyanon, T.; Chiappori, A.; Williams, C.; Simon, G.; Antonia, S.; Gray, J.; Litschauer, S.; Tetteh, L.; Neuger, A.; et al. Phase I/II study of the Src inhibitor Dasatinib in combination with Erlotinib in advanced non-small-cell lung cancer. J. Clin. Oncol. 2010, 28, 1387–1394. [Google Scholar] [CrossRef]
- Berger, T.G.; Dieckmann, D.; Efferth, T.; Schultz, E.S.; Funk, J.O.; Bau, A.; Schuler, G. Artesunate in the treatment of metastatic uveal melanoma—First experiences. Oncol. Rep. 2005, 14, 1599–1603. [Google Scholar]
- Meshnick, S.R.; Yang, Y.Z.; Lima, V.; Kuypers, F.; Kamchonwongpaisan, S.; Yuthavong, Y. Iron-dependent free radical generation from the antimalarial agent artemisinin (qinghaosu). Antimicrob. Agents Chemother. 1993, 37, 1108–1114. [Google Scholar]
- Xu, H.; He, Y.; Yang, X.; Liang, L.; Zhan, Z.; Ye, Y.; Yang, X.; Lian, F.; Sun, L. Anti-malarial agent artesunate inhibits TNF-alphainduced production of proinflammatory cytokines via inhibition of NF-kappaB and PI3 kinase/Akt signal pathway in human rheumatoid arthritis fibroblast-like synoviocytes. Rheumatology 2007, 46, 920–926. [Google Scholar] [CrossRef]
- Rasheed, S.A.; Efferth, T.; Asangani, I.A.; Allgayer, H. First evidence that the antimalarial drug Artesunate inhibits invasion and in vivo metastasis in lung cancer by targeting essential extracellular proteases. Int. J. Cancer 2010, 127, 1475–1485. [Google Scholar] [CrossRef]
- Li, S.; Xue, F.; Cheng, Z.; Yang, X.; Wang, S.; Geng, F.; Pan, L. Effect of artesunate on inhibiting proliferation and inducing apoptosis of SP2/0 myeloma cells through affecting NFkappaB p65. Int. J. Hematol. 2009, 90, 513–521. [Google Scholar] [CrossRef]
- Hou, J.; Wang, D.; Zhang, R.; Wang, H. Experimental therapy of hepatoma with artemisinin and its derivatives: In vitro and in vivo activity, chemosensitization, and mechanisms of action. Clin. Cancer Res. 2008, 14, 519–5530. [Google Scholar]
- Miao, L.Y.; Zhang, Z.Y. Reversal effect of artesunate on the multidrug-resistance of human leukemia K562/A02 cells. J. Southeast Univ. 2006, 25, 445–447. [Google Scholar]
- Hynes, N.E.; Lane, H.A. ERBB receptors and cancer: The complexity of targeted inhibitors. Nat. Rev. Cancer 2005, 5, 341–354. [Google Scholar] [CrossRef]
- Citri, A.; Yarden, Y. EGF-ERBB signaling: Towards the systems level. Nat. Rev. Mol. Cell Biol. 2006, 7, 505–516. [Google Scholar] [CrossRef]
- Alaoui-Jamali, M.A.; He, Q. The interface between ErbB and non-ErbB receptors in tumor invasion: Clinical implications and opportunities for target discovery. Drug Resist. Update. 2003, 6, 95–107. [Google Scholar] [CrossRef]
- Salomon, D.S.; Brandt, R.; Ciardiello, F.; Normanno, N. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit. Rev. Oncol./Hematol. 1995, 19, 183–232. [Google Scholar] [CrossRef]
- Gottesman, M.M.; Fojo, T.; Bates, S.E. Multidrug resistance in cancer: Role of ATP dependent transporters. Nat. Rev. Cancer 2002, 2, 48–58. [Google Scholar] [CrossRef]
- Hirschmann-Jax, C.; Foster, A.E.; Wulf, G.G.; Nuchtern, J.G.; Jax, T.W.; Gobel, U.; Goodell, M.A.; Brenner, M.K. A distinct ‘side population’ of cells with high drug efflux capacity in human tumor cells. Proc. Natl. Acad. Sci. USA 2004, 101, 14228–14233. [Google Scholar]
- Rabindran, S.K.; Ross, D.D.; Doyle, L.A.; Yang, W.; Greenberger, L.M. Fumitremorgin C reverses multidrug resistance in cells transfected with the breast cancer resistance protein. Cancer Res. 2000, 60, 47–50. [Google Scholar]
- Yin, L.; Castagnino, P.; Assoian, R.K. ABCG2 expression and side population abundance regulated by a transforming growth factor beta-directed epithelial-mesenchymal transition. Cancer Res. 2008, 68, 800–807. [Google Scholar] [CrossRef]
- Evseenko, D.A.; Paxton, J.W.; Keelan, J.A. Independent regulation of apical and basolateral drug transporter expression and function in placental trophoblasts by cytokines, steroids, and growth factors. Drug Metab. Dispos. 2007, 35, 595–601. [Google Scholar] [CrossRef]
- Mogi, M.; Yang, J.; Lambert, J.F.; Colvin, G.A.; Shiojima, I.; Skurk, C.; Summer, R.; Fine, A.; Quesenberry, P.J.; Walsh, K. Akt signaling regulates side population cell phenotype via Bcrp1 translocation. J. Biol. Chem. 2003, 278, 39068–39075. [Google Scholar]
- Takada, T.; Suzuki, H.; Gotoh, Y.; Sugiyama, Y. Regulation of the cell surface expression of human BCRP/ABCG2 by the phosphorylation state of Akt in polarized cells. Drug Metab. Dispos. 2005, 33, 905–909. [Google Scholar] [CrossRef]
- Sertel, S.; Eichhornb, T.; Sieberb, S.; Sauerc, A.; Weissc, J.; Plinkerta, P.K.; Efferth, T. Factors determining sensitivity or resistance of tumor cell lines towards artesunate. Chem.-Biol. Interact. 2010, 185, 42–52. [Google Scholar] [CrossRef]
- Liu, W.M. Enhancing the cytotoxic activity of novel targeted therapies—Is there a role for a combinatorial approach? Curr. Clin. Pharmacol. 2008, 3, 108–117. [Google Scholar] [CrossRef]
- Liu, WM; Gravett, A.M.; Dalgleish, A.G. The antimalarial agent artesunate possesses anticancer properties that can be enhanced by combination strategies. Int. J. Cancer 2011, 128, 1471–1480. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ma, H.; Yao, Q.; Zhang, A.-M.; Lin, S.; Wang, X.-X.; Wu, L.; Sun, J.-G.; Chen, Z.-T. The Effects of Artesunate on the Expression of EGFR and ABCG2 in A549 Human Lung Cancer Cells and a Xenograft Model. Molecules 2011, 16, 10556-10569. https://doi.org/10.3390/molecules161210556
Ma H, Yao Q, Zhang A-M, Lin S, Wang X-X, Wu L, Sun J-G, Chen Z-T. The Effects of Artesunate on the Expression of EGFR and ABCG2 in A549 Human Lung Cancer Cells and a Xenograft Model. Molecules. 2011; 16(12):10556-10569. https://doi.org/10.3390/molecules161210556
Chicago/Turabian StyleMa, Hu, Quan Yao, An-Mei Zhang, Sheng Lin, Xin-Xin Wang, Lei Wu, Jian-Guo Sun, and Zheng-Tang Chen. 2011. "The Effects of Artesunate on the Expression of EGFR and ABCG2 in A549 Human Lung Cancer Cells and a Xenograft Model" Molecules 16, no. 12: 10556-10569. https://doi.org/10.3390/molecules161210556