In Vitro Inhibitory Effects of Cyandin-3-rutinoside on Pancreatic α-Amylase and Its Combined Effect with Acarbose
Abstract
:1. Introduction
2. Results and Discussion
2.1. The IC50 values for pancreatic α-amylase
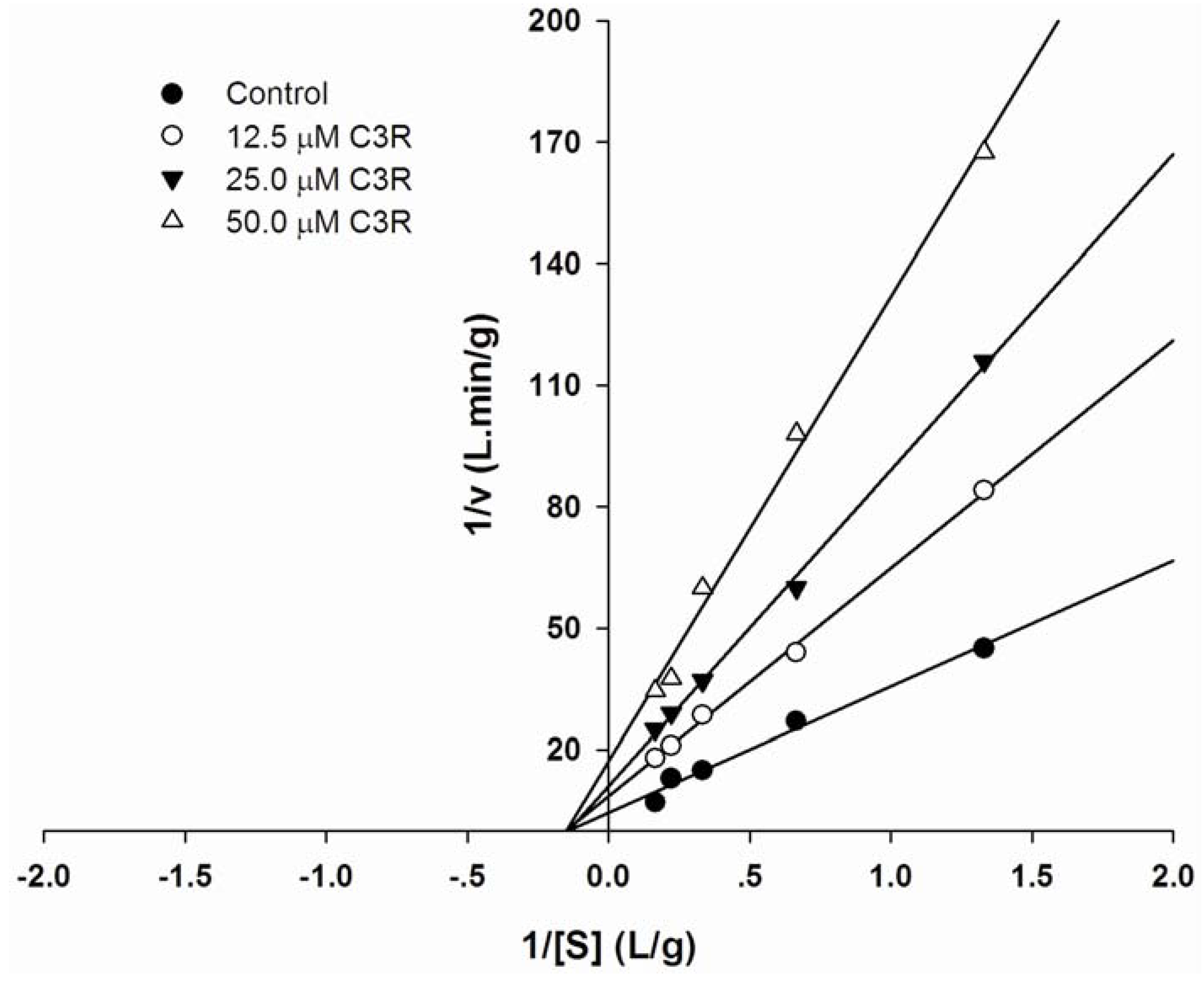
2.2. The type of inhibition of cyanidin-3-rutinoside on pancreatic α-amylase
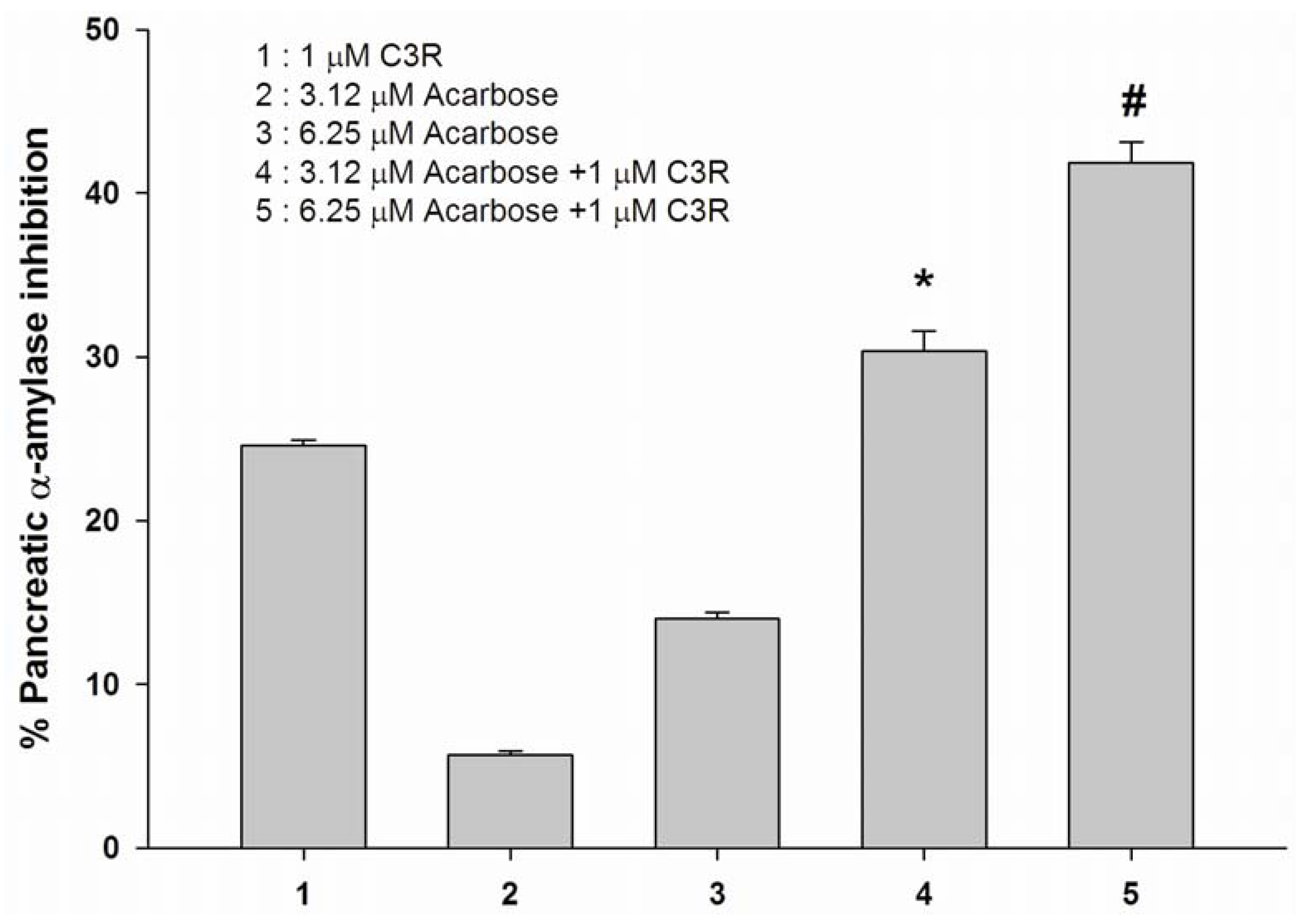
2.3. The combined effect of cyanidin-3-rutinoside with acarbose on inhibition of pancreatic α-amylase activity in vitro
3. Experimental
3.1. Chemicals
3.2. Pancreatic α-amylase inhibition assay
3.3. Enzyme kinetics
3.4. Combined inhibitory effect of cyanidin-3-rutinoside and acarbose
3.5. Statistical analysis
4. Conclusions
Acknowledgements
References and Notes
- Tundis, R.; Loizzo, M.R.; Menichini, F. Natural products as alpha-amylase and alpha-glucosidase inhibitors and their hypoglycaemic potential in the treatment of diabetes: An update. Mini Rev. Med. Chem. 2010, 10, 315–331. [Google Scholar] [CrossRef] [PubMed]
- Chiasson, J.L.; Josse, R.G.; Gomis, R.; Hanefeld, M.; Karasik, A.; Laakso, M. STOP-NIDDM trial research group. Acarbose for the prevention of Type 2 diabetes, hypertension and cardiovascular disease in subjects with impaired glucose tolerance: Facts and interpretations concerning the critical analysis of the STOP-NIDDM Trial data. Lancet 2002, 359, 2072–2077. [Google Scholar] [CrossRef]
- Kalt, W.; Hanneken, A.; Milbury, P.; Tremblay, F. Recent research on polyphenolics in vision and eye health. J. Agric. Food Chem. 2010, 58, 4001–4007. [Google Scholar] [CrossRef] [PubMed]
- Laokuldilok, T.; Shoemaker, C.F.; Jongkaewwattana, S.; Tulyathan, V. Antioxidants and antioxidant activity of several pigmented rice brans. J. Agric. Food Chem. 2011, 59, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.S.; Stoner, G.D. Anthocyanins and their role in cancer prevention. Cancer Lett. 2008, 269, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Min, S.W.; Ryu, S.N.; Kim, D.H. Anti-inflammatory effects of black rice, cyanidin-3-O-beta-D-glycoside, and its metabolites, cyanidin and protocatechuic acid. Int. Immunopharmacol. 2010, 10, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Takikawa, M.; Inoue, S.; Horio, F.; Tsuda, T. Dietary anthocyanin-rich bilberry extract ameliorates hyperglycemia and insulin sensitivity via activation of AMP-activated protein kinase in diabetic mice. J. Nutr. 2010, 140, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Ordaz-Galindo, A.; Wesche-Ebeling, P.; Wrolstad, R.E.; Rodriguez-Saona, R.; Argaiz-Jamet, A. Purification and identification of Capulin (Prunus serotina Ehrh) anthocyanins. Food Chem. 1999, 65, 201–206. [Google Scholar] [CrossRef]
- Liu, L.; Cao, S.; Xie, B.; Sun, Z.; Wu, J. Degradation of cyanidin 3-rutinoside in the presence of (-)-epicatechin and litchi pericarp polyphenol oxidase. J. Agric. Food Chem. 2007, 55, 9074–9078. [Google Scholar] [CrossRef] [PubMed]
- Rubinskiene, M.; Jasutiene, I.; Venskutonis, P.R.; Viskelis, P. HPLC determination of the composition and stability of blackcurrant anthocyanins. J. Chromatogr. Sci. 2005, 43, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Ni, H.M.; Wang, S.Y.; Tourkova, I.L.; Shurin, M.R.; Harada, H.; Yin, X.M. Cyanidin-3-rutinoside, a natural polyphenol antioxidant, selectively kills leukemic cells by induction of oxidative stress. J. Biol. Chem. 2007, 282, 13468–13476. [Google Scholar] [CrossRef] [PubMed]
- Adisakwattana, S.; Ngamrojanavanich, N.; Kalampakorn, K.; Tiravanit, W.; Roengsumran, S.; Yibchok-Anun, S. Inhibitory activity of cyanidin-3-rutinoside on alpha-glucosidase. J. Enzyme Inhib. Med. Chem. 2004, 19, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Adisakwattana, S.; Yibchok-Anun, S.; Charoenlertkul, P.; Wongsasiripat, N. Cyanidin-3-rutinoside alleviates postprandial hyperglycemia and its synergism with acarbose by inhibition of intestinal α-glucosidase. J. Clin. Biochem. Nutr. 2010, in press. [Google Scholar] [CrossRef] [PubMed]
- Akkarachiyasit, S.; Charoenlertkul, P.; Yibchok-Anun, S.; Adisakwattana, S. Inhibitory activities of cyanidin and its glycosides and synergistic effect with acarbose against intestinal α-glucosidase and pancreatic α-amylase. Int. J. Mol. Sci. 2010, 11, 3387–3396. [Google Scholar] [CrossRef] [PubMed]
- Kähkönen, M.P.; Heinonen, M. Antioxidant activity of anthocyanins and their aglycons. J. Agric. Food Chem. 2003, 51, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Lebovitz, H.E. Postprandial hyperglycaemic state: importance and consequences. Diabetes Res. Clin. Pract. 1998, 40 (Suppl.), S27–S28. [Google Scholar] [CrossRef]
- Matsumoto, H.; Inaba, H.; Kishi, M.; Tominaga, S.; Hirayama, M.; Tsuda, T. Orally administered delphinidin 3-rutinoside and cyanidin 3-rutinoside are directly absorbed in rats and humans and appear in the blood as the intact forms. J. Agric. Food Chem. 2001, 49, 1546–1551. [Google Scholar] [CrossRef] [PubMed]
- Mulabagal, V.; Lang, G.A.; De Witt, D.L.; Dalavoy, S.S.; Nair, M.G. Anthocyanin content, lipid peroxidation and cyclooxygenase enzyme inhibitory activities of sweet and sour cherries. J. Agric. Food Chem. 2009, 57, 1239–1246. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Li, Y.; Niu, W.; Ding, Y.; Zhang, R.; Shang, X. Analysis and characterisation of anthocyanins in mulberry fruit. Czech J. Food Sci. 2010, 28, 117–126. [Google Scholar] [CrossRef]
- Lo Piparo, E.; Scheib, H.; Frei, N.; Williamson, G.; Grigorov, M.; Chou, C.J. Flavonoids for controlling starch digestion: structural requirements for inhibiting human alpha-amylase. J. Med. Chem. 2008, 51, 3555–3561. [Google Scholar] [CrossRef] [PubMed]
- Chiasson, J.L. Acarbose for the prevention of diabetes, hypertension, and cardiovascular disease in subjects with impaired glucose tolerance: The Study to Prevent Non-Insulin-Dependent Diabetes Mellitus (STOP-NIDDM) Trial. Endocr. Pract. 2006, 12 (Suppl. 1), 25–30. [Google Scholar] [CrossRef]
- Hanefeld, M.; Fischer, S.; Schulze, J.; Spengler, M.; Wargenau, M.; Schollberg, K.; Fücker, K. Therapeutic potentials of acarbose as first-line drug in NIDDM insufficiently treated with diet alone. Diabetes Care 2001, 14, 732–737. [Google Scholar] [CrossRef]
- van de Laar, F.A.; Lucassen, P.L.; Akkermans, R.P.; van de Lisdonk, E.H.; Rutten, G.E.; van Weel, C. Alpha-glucosidase inhibitors for patients with type 2 diabetes: results from a Cochrane systematic review and meta-analysis. Diabetes Care 2005, 28, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Elhabiri, M.; Figueiredo, P.; Fougerousse, A.; Brouillard, R. convenient method for conversion of flavonoids into anthocyanins. Tetrahedron Lett. 1995, 36, 4611–4614. [Google Scholar]
- Kandra, L.; Zajácz, A.; Remenyik, J.; Gyémánt, G. Kinetic investigation of a new inhibitor for human salivary alpha-amylase. Biochem. Biophys. Res. Commun. 2005, 334, 824–828. [Google Scholar] [CrossRef] [PubMed]

| Concentration of cyanidin-3-rutinoside (αM) | |||||||
| 0.1 | 1.0 | 10.0 | 100.0 | 250.0 | 500.0 | 1000.0 | |
| % Inhibition | 0.8 ± 0.1 | 23.7 ± 0.2 | 44.3 ± 0.1 | 52.1 ± 0.2 | 64.1 ± 0.3 | 68.1 ± 0.2 | 70.3 ± 0.3 |
| Compounds | IC50 values (μM) | ||
|---|---|---|---|
| Pancreatic α-amylase | Maltase | Sucrase | |
| Cyanidin-3-rutinoside | 24.4 ± 0.1 | 2,323 ± 14.8 a | 250.2 ± 8.1 a |
| Acarbose | 18.1 ± 0.1 | 2.7 ± 0.1 a | 29.6 ± 3.5 a |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Akkarachiyasit, S.; Yibchok-Anun, S.; Wacharasindhu, S.; Adisakwattana, S. In Vitro Inhibitory Effects of Cyandin-3-rutinoside on Pancreatic α-Amylase and Its Combined Effect with Acarbose. Molecules 2011, 16, 2075-2083. https://doi.org/10.3390/molecules16032075
Akkarachiyasit S, Yibchok-Anun S, Wacharasindhu S, Adisakwattana S. In Vitro Inhibitory Effects of Cyandin-3-rutinoside on Pancreatic α-Amylase and Its Combined Effect with Acarbose. Molecules. 2011; 16(3):2075-2083. https://doi.org/10.3390/molecules16032075
Chicago/Turabian StyleAkkarachiyasit, Sarinya, Sirintorn Yibchok-Anun, Sumrit Wacharasindhu, and Sirichai Adisakwattana. 2011. "In Vitro Inhibitory Effects of Cyandin-3-rutinoside on Pancreatic α-Amylase and Its Combined Effect with Acarbose" Molecules 16, no. 3: 2075-2083. https://doi.org/10.3390/molecules16032075






