Antioxidant and Antityrosinase Activities of Various Extracts from the Fruiting Bodies of Lentinus lepideus
Abstract
:1. Introduction
2. Results and Discussion
2.1. Antioxidant activity on β-carotene-linoleic acid
2.2. Reducing power
2.3. Scavenging effect on DPPH
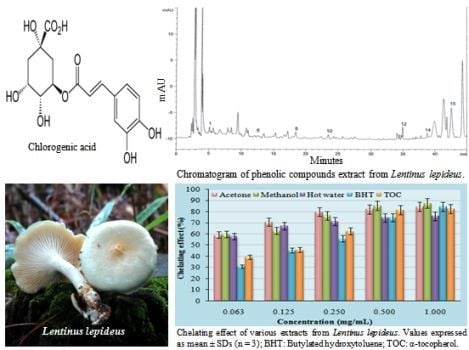
2.4. Chelating effects on ferrous ions
2.5. Analysis of phenolic compound
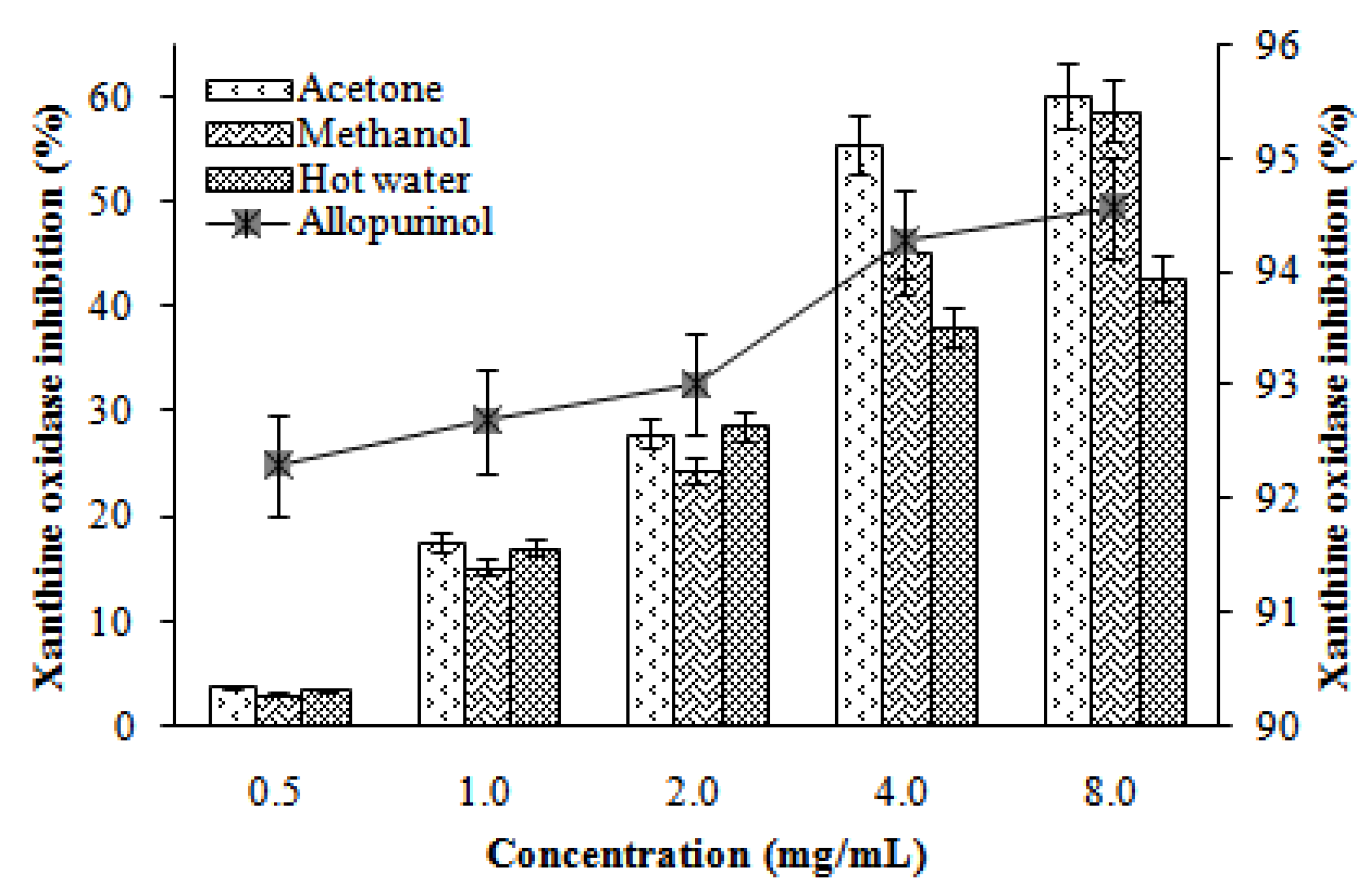
2.6. Xanthine oxidase inhibitory activity
2.7. Tyrosinase inhibition
3. Experimental
3.1. Chemicals and reagents
3.2. Mushroom and extraction
3.3. Antioxidant activity by β-carotene-linoleic acid
3.4. Reducing power
3.5. Scavenging effect on 1,1-diphenyl-2-picrylhydrazyl radicals
3.6. Chelating effects on ferrous ions
3.7. Analysis of phenolic compounds
3.8. Xanthine oxidase inhibition
3.9. Tyrosinase inhibition
3.10. Statistical analysis
4. Conclusions
Acknowledgements
References
- Hibbett, D.S.; Vilgalys, R. Phylogenetic relationships of Lentinus (Basidiomycotina) inferred from molecular and morphological characters. Syst. Bot. 1993, 18, 409–433. [Google Scholar] [CrossRef]
- Mattila, P.; Konko, K.; Eurola, M.; Pihlava, J.M.; Astola, J.; Vahteristo, L.; Hietaniemi, V.; Kumpulainen, J.; Valtonen, M.; Piironen, V. Contents of vitamins, mineral elements, and some polyphenolic compounds in cultivated mushrooms. J. Agric. Food Chem. 2001, 49, 2343–2348. [Google Scholar] [CrossRef] [PubMed]
- Cheung, L.M.; Cheung, P.C.; Ooi, V.E. Antioxidant activity and total phenolics of edible mushroom extracts. Food Chem. 2003, 81, 249–255. [Google Scholar] [CrossRef]
- Fukushoma, M.; Ohashi, T.; Fujiwara, Y.; Sonoyama, K.; Nakano, M. Cholesterol-lowering effects of maitake (Grifola frondosa) fiber, shiitake (Lentinus edodes) fiber, enokitake (Flammulina velutipes) fiber in rats. Exp. Biol. Med. 2001, 226, 758–765. [Google Scholar] [CrossRef]
- Velioglu, Y.S.; Mazza, G.; Gao, L.; Oomah, B.D. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J. Agric. Food Chem. 1998, 46, 4113–4117. [Google Scholar] [CrossRef]
- Turkoglu, A.; Duru, M.E.; Mercan, N.; Kivrak, I.; Gezer, K. Antioxidant and antimicrobial activities of Laetiporus sulphureus (Bull.) Murrill. Food Chem. 2007, 101, 267–273. [Google Scholar] [CrossRef]
- Zhou, C.X.; Kong, L.D.; Ye, W.C.; Cheng, C.H.; Tan, R.X. Inhibition of xanthine and monoamine oxidases by stillbenoids from Veratrum taliense. Planta Med. 2001, 67, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.S.; Lee, Y.J.; Lu, F.J.; Chaing, H.C. Inhibitory effects of flavonoids on xanthine oxidase. Anticancer Res. 1993, 13, 2165–2170. [Google Scholar] [PubMed]
- Hearing, V.J.; Jimenez, M. Mammalian tyrosinase- the critical regulatory control point in melanocyte pigmentation. Int. J. Biochem. 1987, 19, 1141–1147. [Google Scholar] [CrossRef]
- Barros, L.; Ferreira, M.J.; Queiros, B.; Ferreira, I.C.; Bapista, P. Total phenols, ascorbic acid, β-carotene and lycopene in Portuguese wild edible mushrooms and their antioxidant activities. Food Chem. 2007, 103, 413–419. [Google Scholar] [CrossRef]
- Lee, Y.L.; Huang, G.W.; Liang, Z.C.; Mau, J.L. Antioxidant properties of three extracts from Pleurotus citrinopileatus. LWT-Food Sci. Technol. 2007, 40, 823–833. [Google Scholar] [CrossRef]
- Shimada, K.; Fujikawa, K.; Yahara, K.; Nakamura, T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J. Agric. Food Chem. 1992, 40, 945–948. [Google Scholar] [CrossRef]
- Herraiz, T.; Galisteo, J.; Chamorro, C. L-tryptophan reacts with naturally occurring and food-occurring phenolic aldehydes to give phenolic tetrahydro-β-caroline alkaloids: Activity as antioxidants and free radical scavengers. J. Agric. Food Chem. 2003, 51, 2168–2173. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.Y.; Huang, S.J.; Mau, J.L. Antioxidant properties of hot water extracts from Agrocybe cylindracea. Food Chem. 2006, 98, 670–677. [Google Scholar] [CrossRef]
- Lee, Y.L.; Yen, M.T.; Mau, J.L. Antioxidant properties of various extracts from Hypsizigus marmoreus. Food Chem. 2007, 104, 1–9. [Google Scholar] [CrossRef]
- Yamaguchi, R.; Tatsumi, Y.; Asano, M.; Kato, K.; Ueno, Y. Effect of metal salts and fructose on the autoxidation of methyl linoleate in emulsions. Agric. Biol. Chem. 1988, 52, 849–850. [Google Scholar]
- Kim, M.Y.; Seguin, P.; Ahn, J.K.; Kim, J.J.; Chun, S.C.; Kim, E.H.; Seo, S.H.; Kang, E.Y.; Kim, S.L.; Park, Y.J.; Ro, H.M.; Chung, I.M. Phenolic compound concentration and antioxidant activities of edible and medicinal mushrooms from Korea. J. Agric. Food Chem. 2008, 56, 7265–7270. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Wang, K.; Huang, S.; Wang, H.; Mu, X.; He, C.; Ji, X.; Zhang, J.; Huang, F. Antioxidant activity of microwave-assisted extract of longan (Dimocarpus longan Lour.) peel. Food Chem. 2008, 106, 1264–1270. [Google Scholar] [CrossRef]
- Prasad, K.N.; Divakar, S.; Shivamurthy, G.R.; Aradhya, S.M. Isolation of a free radical scavenging antioxidant from water spinach (Ipomoea aquatica Forsk). J. Sci. Food Agric. 2005, 85, 1461–1468. [Google Scholar] [CrossRef]
- Costantino, L.; Albasini, A.; Rastelli, G.; Benvenuti, S. Activity of polyphenolic crude extracts as scavengers of superoxide radicals and inhibitors of xanthine oxidase. Planta Med. 1992, 58, 342–344. [Google Scholar] [CrossRef] [PubMed]
- Baek, H.S.; Rho, H.S.; Yoo, J.W.; Ahn, S.M.; Lee, J.Y.; Lee, J.; Kim, M.K.; Kim, D.H.; Chang, I.S. The inhibitory effect of new hydroxamic acid derivatives on melanogenesis. Bull. Korean Chem. Soc. 2008, 29, 43–46. [Google Scholar]
- Kubo, I.; Chen, Q.X.; Nihei, K.I. Molecular design of antibrowning agents: antioxidative tyrosinase inhibitors. Food Chem. 2003, 81, 241–247. [Google Scholar] [CrossRef]
- Momtaz, S.; Mapunya, B.M.; Houghton, P.J.; Edgerly, C.; Hussein, A.; Naidoo, S.; Lall, N. Tyrosinase inhibition by extracts and constituents of Sideroxylon inerme L. stem bark, used in South Africa for skin lightening. J. Ethnopharmacol. 2008, 119, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Dapkevicius, A.; Venskutonis, R.; van Beek, T.A.; Linssen, J.P. Antioxidant activity of extracts obtained by different isolation procedures from some aromatic herbs grown in Lithuania. J. Sci. Food Agric. 1998, 77, 140–146. [Google Scholar] [CrossRef]
- Gulcin, I.; Buyukokuroglu, M.E.; Oktay, M.; Kufrevioglu, O.I. Antioxidant and analgesic activities of turpentine of Pinus nigra Arn. subsp. pallsiana (Lamb.) Holmboe. J. Ethnopharmacol. 2003, 86, 51–58. [Google Scholar] [CrossRef]
- Cuendet, M.; Hostettmann, K.; Potterat, O.; Dyatmiko, W. Iridoid glucosides with free radical scavenging properties from Fagraea blumei. Helv. Chim. Acta 1997, 80, 1144–1152. [Google Scholar] [CrossRef]
- Dinis, T.C.; Madeira, V.M.; Almeida, L.M. Action of phenolic derivatives (acetaminophen, salicylate, and 5-amino salicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch. Biochem. Biophys. 1994, 315, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.; Kim, S.H.; Chung, J.I.; Chi, H.Y.; Kim, J.A.; Chung, I.M. Analysis phenolic compounds and isoflavones in soybean seeds (Glycine max (L.) Merill) and sprouts grown under different conditions. Eur. Food Res. Technol. 2006, 222, 201–208. [Google Scholar] [CrossRef]
- Owen, P.L.; Johns, T. Xanthine oxidase inhibitory activity of North-Eastern North American plant remedies used for gout. J. Ethnopharmacol. 1999, 64, 149–160. [Google Scholar] [CrossRef]
- Masuda, T.; Yamashita, D.; Takeda, Y.; Yonemori, S. Screening for tyrosinase inhibitors among extracts of seashore plants and identification of potent inhibitors from Garcinia subelliptica. Biosci. Biotechnol. Biochem. 2005, 69, 197–201. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the Lentinus lepideus, IUM-4459 are available from the CCDBM, Division of Life Sciences, University of Incheon. |




| Solvent and control | Sample concentration (mg/mL) | |||
|---|---|---|---|---|
| 0.5 | 2.0 | 8.0 | 20.0 | |
| Acetone | 68.48 ± 0.59 c | 86.74 ± 0.23 a | 92.63 ± 0.18 b | 95.52 ± 0.37 a |
| Methanol | 59.69 ± 0.71 d | 85.03 ± 0.67 a,b | 91.23 ± 0.53 b | 95.42 ± 0.02 a |
| Hot water | 68.73 ± 0.32 c | 88.12 ± 0.99 a | 96.32 ± 0.25 a | 96.33 ± 0.86 a |
| BHT | 95.21 ± 0.17 a | - | - | - |
| TOC | 96.02 ± 0.18 a | - | - | - |
| Solvent and control | Sample concentration (mg/mL) | |||
|---|---|---|---|---|
| 1.0 | 2.0 | 4.0 | 8.0 | |
| Acetone | 0.31 ± 0.03 e | 0.46 ± 0.03 ab | 0.73 ± 0.04 b | 1.12 ± 0.05 ab |
| Methanol | 0.36 ± 0.07 e | 0.53 ± 0.11 a | 0.82 ± 0.11 a | 1.21 ± 0.31 a |
| Hot water | 0.32 ± 0.26 e | 0.42 ± 0.22 b | 0.64 ± 0.19 c | 1.10 ± 0.11 ab |
| BHT | 3.21 ± 0.49 a | - | - | - |
| TOC | 2.16 ± 0.32 b | - | - | - |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yoon, K.N.; Alam, N.; Lee, K.R.; Shin, P.G.; Cheong, J.C.; Yoo, Y.B.; Lee, T.S. Antioxidant and Antityrosinase Activities of Various Extracts from the Fruiting Bodies of Lentinus lepideus. Molecules 2011, 16, 2334-2347. https://doi.org/10.3390/molecules16032334
Yoon KN, Alam N, Lee KR, Shin PG, Cheong JC, Yoo YB, Lee TS. Antioxidant and Antityrosinase Activities of Various Extracts from the Fruiting Bodies of Lentinus lepideus. Molecules. 2011; 16(3):2334-2347. https://doi.org/10.3390/molecules16032334
Chicago/Turabian StyleYoon, Ki Nam, Nuhu Alam, Kyung Rim Lee, Pyung Gyun Shin, Jong Chun Cheong, Young Bok Yoo, and Tae Soo Lee. 2011. "Antioxidant and Antityrosinase Activities of Various Extracts from the Fruiting Bodies of Lentinus lepideus" Molecules 16, no. 3: 2334-2347. https://doi.org/10.3390/molecules16032334