Cyclic Water Clusters in Tape-Like and Cage-Like Structures
Abstract
:1. Introduction
2. Results and Discussion
Crystal Structures of Compounds 1-2
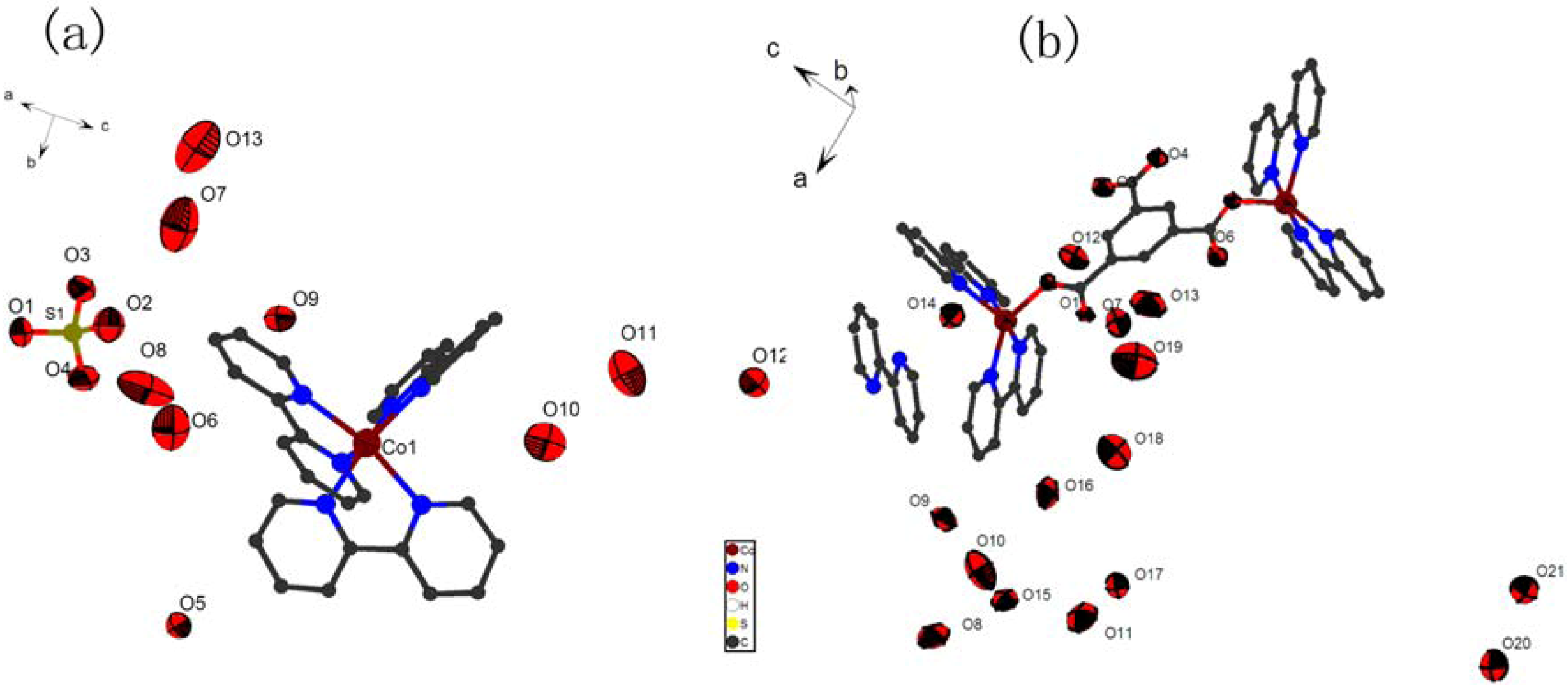
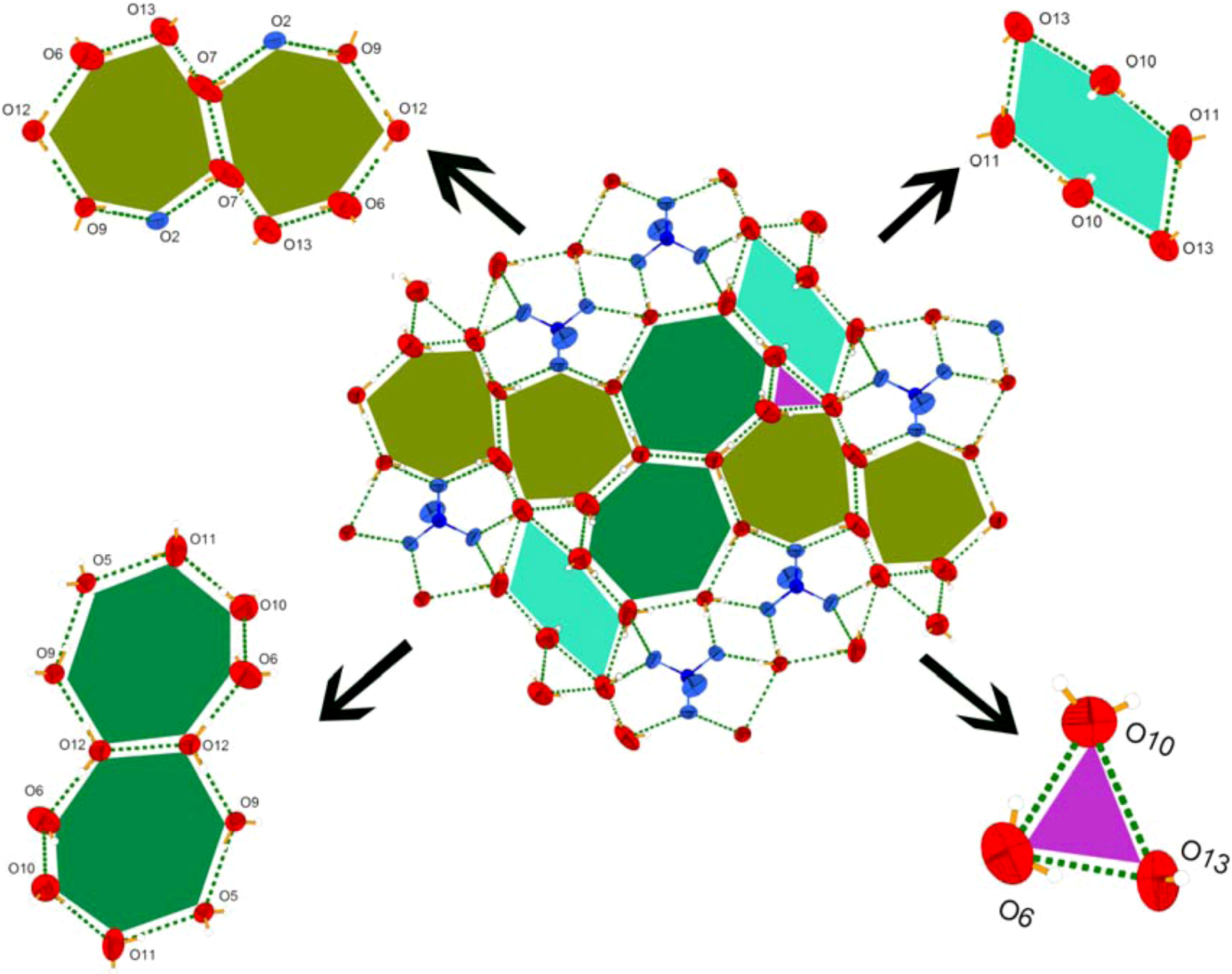
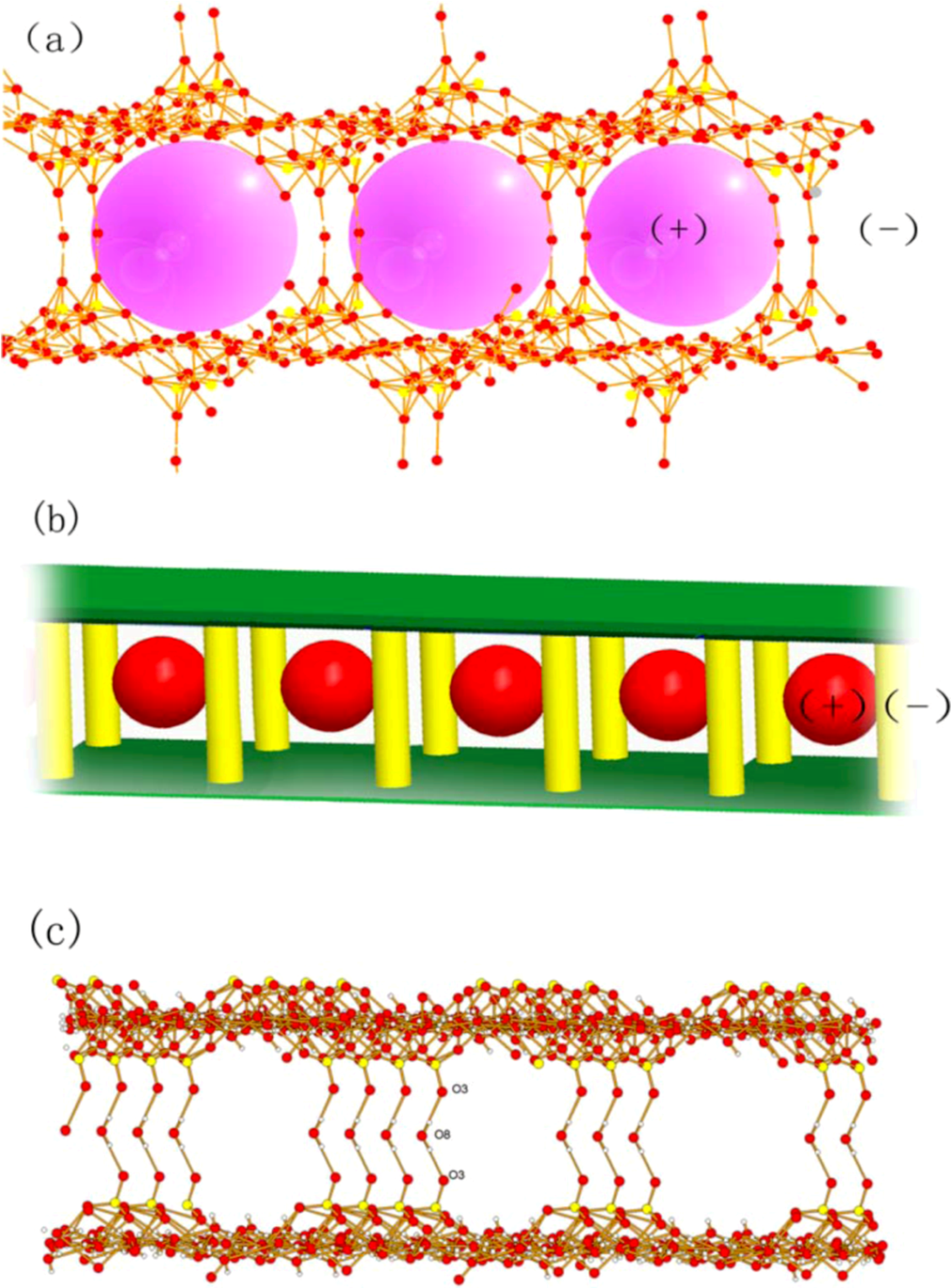
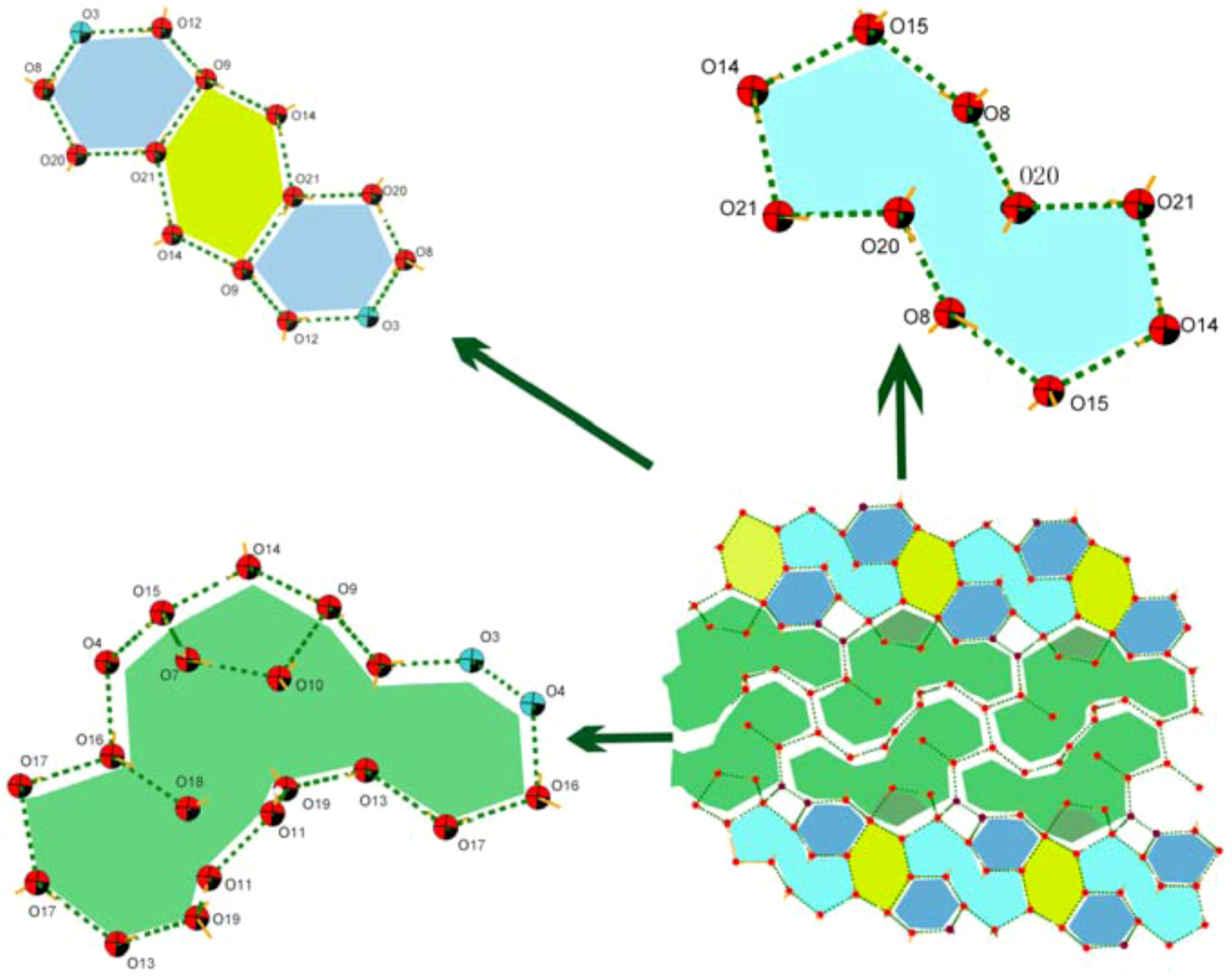

| Complex | 1 | 2 |
| Empirical formula | C60H82Co2N12O25S2 | C54H49Cu2N9O11 |
| Formula weight | 1553.35 | 1127.11 |
| Temperature(K) | 293(2) K | 296(2) K |
| Crystal system | Monoclinic | Monoclinic |
| Space group | C2/c | C2/c |
| a(Å) | 22.979(4) | 12.551(5) |
| b(Å) | 13.656(3) | 23.483(9) |
| c(Å) | 24.890(6) | 42.330(17) |
| α(°) | 90 | 90 |
| β(°) | 115.235(2) | 95.053(6) |
| γ(°) | 90 | 90 |
| V(Å3) | 7065(3) | 12428(9) |
| Z | 4 | 8 |
| Dcalc(mgcm-3) | 1.442 | 1.397 |
| μ(mm-1) | 0.614 | 0.765 |
| F[000] | 3204 | 5456 |
| θ(°) | 1.78 to 25.01 | 0.97 to 25.01 |
| Data/restraints/parameters | 6201/ 0 / 457 | 10944 / 0 / 784 |
| Goodness-of-fit on F2 | 1.020 | 1.045 |
| Fianl Ra indices[I>2σ(I)] | R1 = 0.0609, wR2 = 0.1764 | R1 = 0.0746, wR2 = 0.1983 |
| R indices (all data) | R1 = 0.0724, wR2 = 0.1900 | R1 = 0.1118, wR2 = 0.2244 |
| D-H···A | d(H···A) | d(D···A) | <(DHA) | Symmetry code |
| O(5)-H(5C)...O(1) | 1.97 | 2.819(6) | 172.4 | x-1/2,y+1/2,z |
| O(5)-H(5D)...O(1)z | 1.92 | 2.768(6) | 172.4 | -x+1/2,-y+3/2,-z |
| O(5)-H(5D)...S(1) | 2.96 | 3.771(4) | 160.9 | -x+1/2,-y+3/2,-z |
| O(6)-H(6C)...O(10) | 1.81 | 2.575(11) | 148.6 | x,-y+1,z-1/2 |
| O(6)-H(6D)...O(13) | 1.68 | 2.47(2) | 155.3 | -x+1/2,-y+1/2,-z+1 |
| O(7)-H(7C)...O(2) | 1.98 | 2.792(14) | 160.5 | -x+1/2,-y+1/2,-z+1 |
| O(7)-H(7D)...O(13) | 1.19 | 2.03(3) | 170.2 | |
| O(8)-H(8C)...O(3) | 1.78 | 2.621(8) | 172.9 | |
| O(8)-H(8C)...S(1) | 2.95 | 3.719(3) | 151.5 | |
| O(9)-H(9C)...O(2) | 1.91 | 2.742(7) | 167.3 | -x+1/2,-y+1/2,-z |
| O(9)-H(9D)...O(5) | 2.07 | 2.910(7) | 167.8 | -x,-y+1,-z |
| O(10)-H(10D)...O(11) | 1.97 | 2.815(11) | 174.9 | |
| O(11)-H(11A)...O(13) | 2.19 | 2.89(3) | 139.3 | x,-y,z-1/2 |
| O(11)-H(11C)...O(5) | 2.04 | 2.888(8) | 172.5 | x,-y+1,z+1/2 |
| O(12)-H(12C)...O(6) | 1.88 | 2.728(10) | 179.2 | -x+1/2,-y+1/2,-z+1 |
| O(12)-H(12D)...O(9) | 1.92 | 2.767(7) | 177.8 | -x+1/2,-y+1/2,-z+1 |
| O(13)-H(13C)...O(4) | 1.81 | 2.65(2) | 172.1 | -x+1/2,-y+1/2,-z+1 |
| O(13)-H(13C)...S(1) | 2.83 | 3.58(2) | 148.1 | -x+1/2,-y+1/2,-z+1 |
| Complex 2 | ||||
| O(7)-H(7A)...O(1) | 1.92 | 2.771(8) | 174.3 | |
| O(7)-H(7B)...O(10) | 1.90 | 2.746(14) | 173.1 | x-1,y,z |
| O(8)-H(8A)...O(3) | 1.86 | 2.692(10) | 164.1 | x+1,y-1,z |
| O(8)-H(8B)...O(15) | 1.94 | 2.766(14) | 165.2 | |
| O(9)-H(9A)...O(14) | 1.95 | 2.802(11) | 176.5 | x+1/2,y-1/2,z |
| O(9)-H(9B)...O(12) | 1.97 | 2.767(11) | 156.7 | x+1/2,y-1/2,z |
| O(10)-H(10A)...O(6) | 2.32 | 3.127(14) | 158.6 | x+1,y,z |
| O(10)-H(10B)...O(9) | 2.14 | 2.951(18) | 158.2 | |
| O(11)-H(11A)...O(19) | 1.87 | 2.716(18) | 177.4 | x+1/2,y-1/2,z |
| O(11)-H(11B)...O(6) | 1.93 | 2.776(10) | 177.8 | x+1,y,z |
| O(12)-H(12B)...O(3) | 1.88 | 2.715(10) | 169.0 | |
| O(12)-H(12A)...O(13) | 2.30 | 3.093(15) | 154.8 | |
| O(13)-H(13A)...O(19) | 1.87 | 2.71(2) | 174.0 | |
| O(14)-H(14A)...O(15) | 2.06 | 2.906(12) | 178.6 | x,y+1,z |
| O(14)-H(14B)...O(21) | 1.95 | 2.802(12) | 179.0 | x+1/2,y+1/2,z+1 |
| O(15)-H(15A)...O(4) | 1.93 | 2.775(8) | 176.1 | x+1,y-1,z |
| O(15)-H(15B)...O(7) | 1.88 | 2.726(10) | 176.6 | x+1/2,y-1/2,z |
| O(16)-H(16A)...O(4) | 1.93 | 2.762(9) | 166.4 | x+1/2,y-1/2,z |
| O(16)-H(16B)...O(18) | 1.90 | 2.730(14) | 165.1 | |
| O(17)-H(17A)...O(16) | 2.13 | 2.978(12) | 173.5 | -x+2,y,-z+3/2 |
| O(17)-H(17B)...O(13) | 2.14 | 2.982(13) | 173.3 | -x+1,y,-z+3/2 |
| O(18)-H(18A)...O(19) | 2.33 | 3.15(2) | 163.0 | |
| O(19)-H(19A)...O(18) | 2.61 | 3.15(2) | 122.2 | |
| O(20)-H(20B)...O(8) | 2.22 | 3.067(16) | 177.2 | x-1/2,y+1/2,z-1 |
| O(20)-H(20A)...O(8) | 1.74 | 2.586(15) | 173.3 | -x+3/2,-y+1/2,-z+1 |
| O(21)-H(21A)...O(9) | 2.15 | 2.992(12) | 170.9 | -x+1,-y+1,-z+1 |
| O(21)-H(21B)...O(20) | 1.91 | 2.750(14) | 170.3 |
3. Experimental
3.1. Materials and Physical Measurements
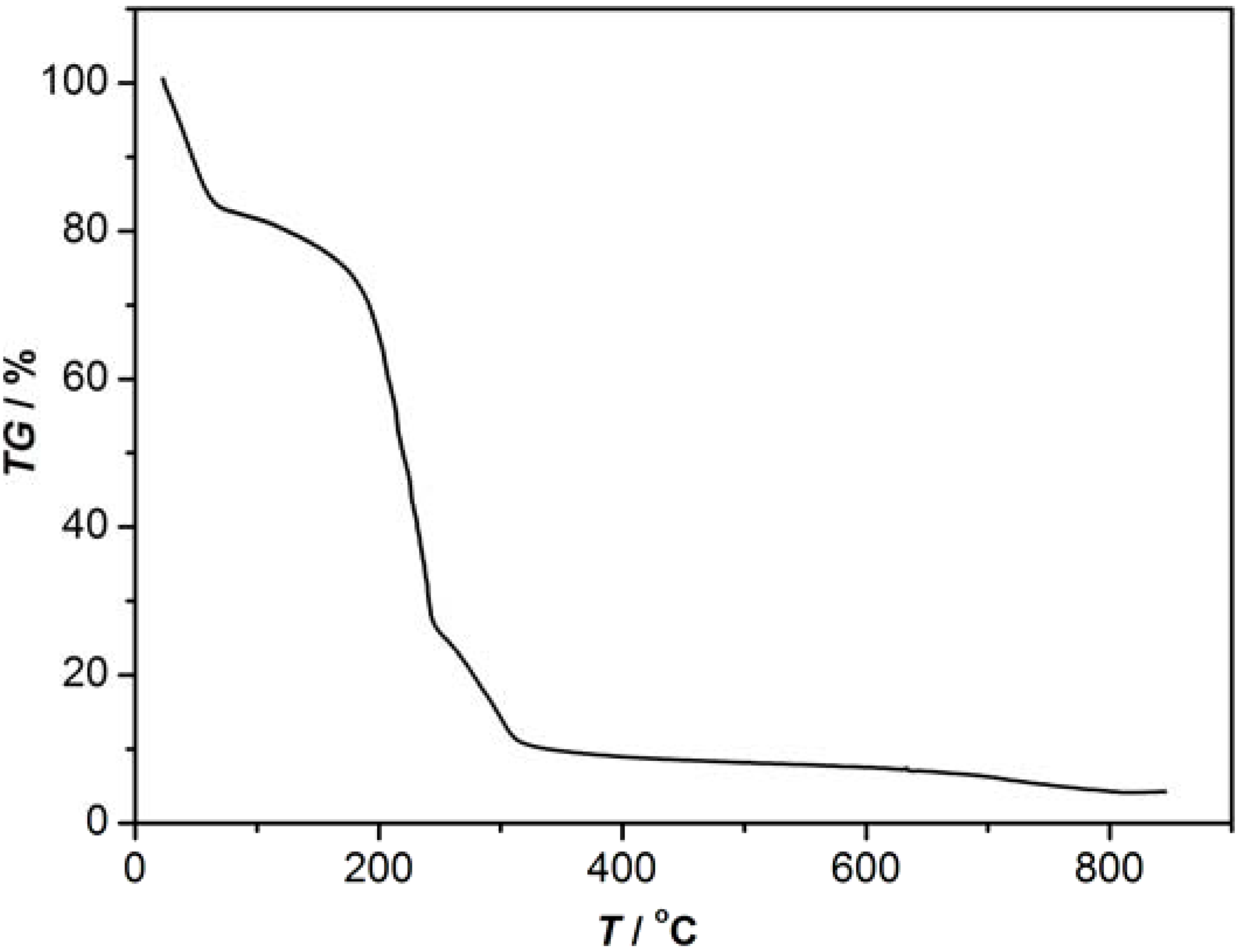
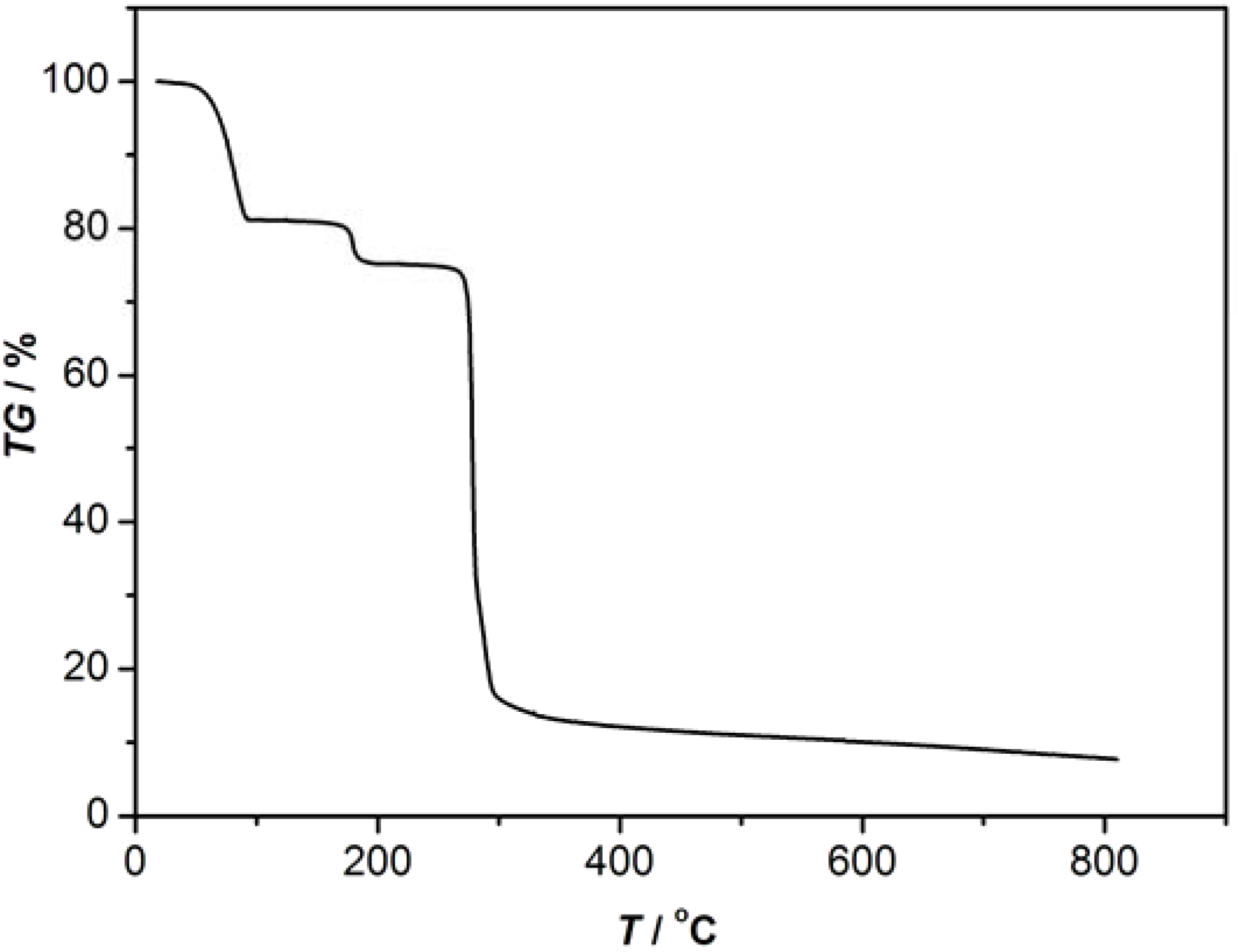
3.2. Synthesis of 1-2
3.2.1. Synthesis of [Co2(2,2′-bpy)6]·(SO4)2·17H2O (1)
3.2.2. Synthesis of [Cu2(2,2′-bpy)4(BTCA)]·(OH)(2,2′-bpy)0.514H2O (2)
3.3. Single-crystal Structure Determination
4. Conclusions
Supplementary Materials
Acknowledgements
References and Notes
- Ludwig, R. Water: From Clusters to the Bulk. Angew. Chem. Int. Ed. Engl. 2001, 40, 1808–1827. [Google Scholar] [CrossRef]
- Xantheas, S.S. Ab initio studies of cyclic water clusters (H2O)n, n = 1-6. II. Analysis of many-body interactions. J. Chem. Phys 1994, 100, 7523–7534. [Google Scholar] [CrossRef]
- Kim, J.; Kim, K.S. Structures, binding energies, and spectra of isoenergetic water hexamer clusters: Extensive ab initio studies. J. Chem. Phys. 1998, 109, 5886–5895. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Bharadwaj, P.K. Coexistence of Water Dimer and Hexamer Clusters in 3D Metal-Organic Framework Structures of Ce(III) and Pr(III) with Pyridine-2,6-dicarboxylic Acid. Inorg. Chem. 2003, 42, 8250–8254. [Google Scholar] [CrossRef]
- Ugalde, J.M.; Alkorta, I.; Elguero, J. Structure and Dynamics of the Host–Guest Complex of a Molecular Tweezer: Coupling Synthesis, Solid-State NMR, and Quantum-Chemical Calculations. Angew. Chem., Int. Ed. 2000, 39, 717–720. [Google Scholar]
- Moorthy, J.N.; Natarajan, R.; Venugopalan, P. Characterization of a Planar Cyclic Form of Water Hexamer in an Organic Supramolecular Complex: An Unusual Self-Assembly of Bimesityl-3,3′-Dicarboxylic Acid. Angew. Chem., Int. Ed. 2002, 41, 3417–3420. [Google Scholar] [CrossRef]
- Naskar, J.P.; Drew, M.G.B.; Hulme, A.; Tocher, D.A.; Datta, D. Occurrence of ribbons of cyclic water pentamers in a metallo-organic framework formed by spontaneous fixation of CO2. CrystEngComm 2005, 7, 67–70. [Google Scholar] [CrossRef]
- Haymet, A.D.J.; Dill, K.A. A Simple Model of Water and the Hydrophobic Effect. J. Am. Chem. Soc. 1998, 120, 3166–3175. [Google Scholar]
- Janiak, C.; Scharmann, T. G.; Mason, S.A. Two-Dimensional Water and Ice Layers: Neutron Diffraction Studies at 278, 263, and 20 K. J. Am. Chem. Soc 2002, 124, 14010–14011. [Google Scholar]
- Baldelli, S.; Schnitzer, C.; Campbell, D.J.; Shultz, M.J. Effect of H2SO4 and Alkali Metal SO4-/H2SO4- Salt Solutions on Surface Water Molecules Using Sum Frequency Generation. J. Phys. Chem. B. 1999, 103, 2789–2795. [Google Scholar] [CrossRef]
- Scatena, L.F.; Brown, M.G.; Richmond, G.L. Water at Hydrophobic Surfaces: Weak Hydrogen Bonding and Strong Orientation Effects. Science 2001, 292, 908–912. [Google Scholar] [CrossRef]
- Rossky, P.J. Chemistry: Only skin-deep. Nature 2002, 419, 889–890. [Google Scholar] [CrossRef]
- Gruenloh, C.J.; Carney, J.R.; Arrington, C.A.; Zwier, T.S.; Fredericks, S.Y.; Jordan, K.D. Infrared Spectrum of a Molecular Ice Cube: The S4 and D2d Water Octamers in Benzene-(Water)8. Science 1997, 276, 1678–1681. [Google Scholar]
- Udachin, K.A.; Ripmeester, J.A. A complex clathrate hydrate structure showing bimodal guest hydration. Nature 1999, 397, 420–423. [Google Scholar]
- Dehl, R.E.; Hoeve, C.A. Broad-Line NMR Study of H2O and D2O in Collagen Fibers. J. Chem. Phys. 1969, 50, 3245–3251. [Google Scholar]
- Wang, L.Y.; Yang, Y.; Liu, K.; Li, B.L.; Zhang, Y. A New “Opened-Cube” (H2O)10 Cluster and Undulated Water Chain in Porous Metal-Organic Frameworks. Crystal Growth Des. 2008, 8, 3902–3904. [Google Scholar] [CrossRef]
- Zhong, R.Q.; Zou, R.Q.; Du, M.; Takeichi, N.; Xu, Q. Observation of helical water chains reversibly inlayed in magnesium imidazole-4,5-dicarboxylate. CrystEngComm 2008, 10, 1175–1179. [Google Scholar] [CrossRef]
- Colson, S. D.; Dunning, T.H. The Structure of Nature's Solvent: Water. Science 1994, 265, 43–44. [Google Scholar]
- Liu, K.; Cruzan, J.D.; Saykally, R.J. Water Clusters. Science 1996, 271, 929–933. [Google Scholar]
- Barbour, L.J.; Orr, G.W.; Atwood, J.L. Characterization of a well resolved supramolecular ice-like (H2O)10 cluster in the solid state. Chem. Commun. 2000, 10, 859–860. [Google Scholar]
- Supriya, S.; Manikumari, S.; Raghavaiah, P.; Das, S.K. A cyclic supramolecular (H2O)4 cluster in an unusual Fe3 complex that aggregates to {Fe3}n with a zig-zag chainlike structure. New J. Chem. 2003, 27, 218–220. [Google Scholar] [CrossRef]
- Kostakis, G.E.; Abbas, G.; Anson, C.E.; Powell, A.K. A new class of 3-D porous framework: [Ln(H2O)n]3+ ions act as pillars between π-stacked and H-bonded sheets of (m-BDTH)- organic anions in [Ln(H2O)n](m-BDTH)3·9(H2O) (Ln=Pr, n=9, Ln=Gd, n= 8). CrystEngComm 2008, 10, 1117–1119. [Google Scholar] [CrossRef]
- López, M.V.; Zaragoza, G.; Otero, M.; Pedrido, R.; Rama, G.; Bermejo, M.R. Supramolecular Aggregation of Pd(II) Monohelicates Directed by Discrete (H2O)8 Clusters in a 1,4-Diaxially Substituted Hexameric Chairlike Conformation. Cryst. Growth Des. 2008, 8, 2083–2086. [Google Scholar] [CrossRef]
- Ma, B.Q.; Sun, H.L.; Gao, S. Cyclic water pentamer in a tape-like structure. Chem. Commun. 2004, 4, 2220–2221. [Google Scholar]
- Lakshminarayanan, P.S.; Suresh, E.; Ghosh, P. Formation of an Infinite 2D-Layered Water of (H2O)45 Cluster in a Cryptand-Water Supramolecular Complex: A Template Effect. J. Am. Chem. Soc. 2005, 127, 13132–13133. [Google Scholar]
- Marek, J.; Trvnček, Z. Cu2(bipy)4(HBTCA)[HBTCA]·(bipy)13H2O. Acta Crystallogr. 2008, E64, m384. [Google Scholar]
- Xu, F.; You, W.; Huang, W. [Cu(bipy)3]SO4 7.5H2O. Acta Crystallogr. 2009, E65, m129. [Google Scholar]
- Infantes, L.; Chisholm, J.; Motherwell, S. Extended motifs from water and chemical functional groups in organic molecular crystals. CrystEngComm 2003, 5, 480–486. [Google Scholar] [CrossRef]
- Luo, S.; Li, F.; Li, T. The conformation conversion from achirality to chirality of a flexible alkane tetracarboxylate ligand in the metal-organic framework. Inorganic Chem. Commu. 2011, 14, 597–600. [Google Scholar] [CrossRef]
- Liu, R.; Mok, K.; Valiyaveettil, V. Solid-state self-assembly of a complex from 1,3,5-benzenetri-carboxylic acid and 1,3,5-trihydroxybenzene : Influence of strong O-H···O and C-H···O hydrogen bonds. New J. Chem. 2001, 25, 890–892. [Google Scholar] [CrossRef]
- Zhang, W.; Bruda, S.; Landee, C.; Parent, J.; Turnbull, M. Structures and magnetic properties of transition metal complexes of 1,3,5-benzenetricarboxylic acid. Inorganica Chimica Acta 2003, 342, 193–201. [Google Scholar] [CrossRef]
- Mei, X.; Wolf, C. Conformational polymorphism of 1,8-dipyridylnaphthalene and encapsulation of chains of fused cyclic water pentamers in a hydrophobic crystal environment. CrystEngComm 2006, 8, 377–380. [Google Scholar] [CrossRef]
- Ma, B.Q.; Sun, H.L.; Gao, S. Formation of Two-Dimensional Supramolecular Icelike Layer Containing (H2O)12 Rings. Angew. Chem. Int. Ed. Engl. 2004, 43, 1374–1376. [Google Scholar] [CrossRef]
- Pal, S.; Sankaran, N.B.; Samsnta, A. Structure of a Self-Assembled Chain of Water Molecules in a Crystal Host. Angew. Chem., Int. Ed 2003, 42, 1741–1743. [Google Scholar] [CrossRef]
- Xiao, W.; Chen, C.; Deng, S.; Mao, X.; Sun, B.; Zhang, N. Supramolecular assembly of [H(4-n)BPTC]n- with pipzH22+: 1D negatively charged rectangular tubes and a 2D neutral fes 4·82 network. CrystEngComm 2010, 12, 2011–2013. [Google Scholar] [CrossRef]
- Sun, J.; Xu, H. Supramolecular assembly of [Co2(2,2′-bpy)6]·(BTCA)·Cl·11H2O: 3D negatively charged cages. Inorg. Chem. Commun. 2011, 14, 254–257. [Google Scholar] [CrossRef]
- Banerjee, S.; Murugavel, R. Formation of One-Dimensional Water inside an Organic Solid: Supramolecular Architectures Derived by the Interaction of Aminobenzoic Acids with Nitrogen Bases and H2SO4. Crystal Growth Des. 2004, 4, 545–552. [Google Scholar] [CrossRef]
- Csajka, F.; Chandler, D. Transition pathways in a many-body system: Application to hydrogen-bond breaking in water. J. Chem. Phys. 1998, 109, 1125–1133. [Google Scholar] [CrossRef]
- Siemens, SAINT: Area Detector Control and Integration Software; Siemens Analytical X-ray Instruments Inc.: Madison, WI, USA, 1996.
- Sheldrick, G.M. SHELXL97 and SHELXTL Software Reference Manual, Vol. 5.1.; Bruker AXS Inc.: Madison, WI, USA, 1997. [Google Scholar]
- Crystallographic data (excluding structure factors) for the structures in this paper have been deposited with the Cambridge Crystallographic Date Centre as supplementary publications CCDC 805059 and 805060 for 1-2, respectively. Copies of the data can be obtained free of charge on application to CCDC, 12 Union Road, Cambridge CB2 1EZ, UK [Fax: 441223-336-033; E-Mail: [email protected].].
- Sample Availability: Samples of the compounds are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Huang, Q.; Diao, L.; Zhang, C.; Lei, F. Cyclic Water Clusters in Tape-Like and Cage-Like Structures. Molecules 2011, 16, 2871-2883. https://doi.org/10.3390/molecules16042871
Huang Q, Diao L, Zhang C, Lei F. Cyclic Water Clusters in Tape-Like and Cage-Like Structures. Molecules. 2011; 16(4):2871-2883. https://doi.org/10.3390/molecules16042871
Chicago/Turabian StyleHuang, Qin, Lihong Diao, Chuang Zhang, and Fuhou Lei. 2011. "Cyclic Water Clusters in Tape-Like and Cage-Like Structures" Molecules 16, no. 4: 2871-2883. https://doi.org/10.3390/molecules16042871




