New Solid Phase Synthesis of Distamycin Analogues
Abstract
:1. Introduction

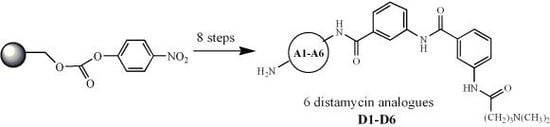
2. Results and Discussion


 | ||||||
|---|---|---|---|---|---|---|
| No. | Substrate A | Fragment R | Yield [%] | Rt | Exact MassFormula | [M+H]+ |
| D1 | A1 |  | 66 | 18.23 | 460.5 | 459.5 |
| 2-amino-5-nitropyridine | C25H28N6O3 | |||||
| D2 | A2 |  | 55 | 18.29 | 461.5 | 460.4 |
| 2-amino-5-nitropyrimidine | C24H27N7O3 | |||||
| D3 | A3 |  | 47 | 18.27 | 474.6 | 473.5 |
| 2-amino-3-methyl-5-nitropyridine | C26H30N6O3 | |||||
| D4 | A4 |  | 49 | 18.24 | 474.6 | 473.5 |
| 2-amino-4-methyl-5-nitropyridine | ||||||
| C26H30N6O3 | ||||||
| D5 | A5 |  | 58 | 18.33 | 466.5 | 465.4 |
| 2-amino-5-nitrotiazole | C23H26N6O3S | |||||
| D6 | A6 |  | 73 | 18.18 | 459.5 | 458.4 |
| 3-nitroaniline | C26H29N5O3 | |||||
3. Experimental
General Procedure
4. Conclusions
Supplementary Materials
Supplementary File 1Acknowledgements
References and Notes
- Arcamone, F.; Penco, P.; Orezzi, P.; Nicolella, V.; Pirelli, A. Structure and synthesis of distamycin A. Nature 1964, 203, 1064–1065. [Google Scholar]
- Bailly, C.; Chaires, J.B. Sequence-specific DNA minor groove binders. Design and synthesis of netropsin and distamycin analouges. Bioconjugate Chem. 1998, 9, 513–538. [Google Scholar]
- Dervan, P.B. Molecular recognition of DNA by small molecules. Bioorg. Med. Chem. 2001, 9, 2215–2235. [Google Scholar] [CrossRef]
- Remers, W.A.; Lyengar, B.S. Antitumor antibiotics. In Cancer Chemotherapeutic Agents, 1th; Foye, W.O., Ed.; American Chemical Society: Washington, DC, USA, 1999; pp. 577–679. [Google Scholar]
- Baraldi, P.G.; Bovero, A.; Fruttarolo, F.; Preti, D.; Tabrizi, M.A.; Pavani, M.G.; Romagnoli, R. DNA minor groove binders as potential antitumor and antimicrobial agents. Med. Res. Rev. 2004, 24, 475–528. [Google Scholar] [CrossRef]
- Boger, L.; Fink, B.E.; Hedrick, M.P. Total syntesis of distamycin A and 2640 analogues: A solution-phase combinatorial approach to the discovery of new, bioactive DNA binding agents and development of rapid, High-Throughput Screen for determining relative DNA binding affinity or DNA binding sequence selectivity. J. Am. Chem. Soc. 2000, 122, 6382–6394. [Google Scholar] [CrossRef]
- Wurtz, N.R.; Turner, J.M.; Baird, E.E.; Dervan, P.B. Fmoc solid phase synthesis of polyamides containing pyrrole and imidazole amino acids. Org. Lett. 2001, 3, 1201–1203. [Google Scholar] [CrossRef]
- Brucoli, F.; Howard., P.W.; Thurston, D.E. Efficient solid-phase synthesis of a library of distamycin analogs containing novel biaryl motifs on SynPhase Lanterns. J. Comb. Chem. 2009, 11, 576–586. [Google Scholar]
- Baraldi, P.G.; Nunez, M.C.; Tabrizi, M.A.; De Clerq, E.; Balzarini, J.; Bermejo, J.; Estévez, F.; Romagnoli, R. Design, synthesis, and biological evaluation of hybrid molecules containing α-methylene-γ-butyrolactones and polypyrrole minor groove binders. J.Med. Chem. 2004, 47, 2877–2886. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Thomas, M. Novel distamycin analogues: facile synthesis of cholesterol conjugates of distamycin-like oligopeptides. Tetrahedron Lett. 2001, 42, 3499–3502. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Thomas, M. Facile synthesis of novel fluorescent distamycin analogues. Tetrahedron Lett. 2001, 42, 5525–5528. [Google Scholar] [CrossRef]
- Tkadlecová, M.; Foltýnová, J.; Valík, M.; Král, V. Spectroscopic binding studies of novel distamycin derivatives. Tetrahedron Lett. 2008, 49, 323–326. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Thomas, M. Facile synthesis of oligopeptide distamycin analogues devoid of hydrogen bond donors or acceptors at the N-terminus: sequence-specific duplex DNA binding as a function of peptide chain length. Tetrahedron Lett. 2000, 41, 5571–5575. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Thomas, M. DNA binding properties of novel distamycin analogs that lack the leading amide unit at the N-terminus. Biochem. Biophys. Res. Commun. 2000, 267, 139–144. [Google Scholar] [CrossRef]
- Thomas, M.; Rao, A.R.; Varshney, U.; Bhattacharya, S. Unusual DNA bonding exhibited by syntheticd istamycin analogues lacking N-terminal amide unit under high salt conditions. J. Biomol. Str. Dyn. 2001, 18, 858–871. [Google Scholar] [CrossRef]
- Thomas, M.; Varshney, U.; Bhattacharya, S. Distamycin analogues without leading amide at their N-termini – comparative binding properties to AT- and GC-rich DNA sequences. Eur. J. Org. Chem. 2002, 3604–3615. [Google Scholar]
- Valík, M.; Malina, J.; Palivec, L.; Foltýnová, J.; Tkadlecová, M.; Urbanová, M.; Brabec, V.; Král, V. Tröger’s base scaffold in racemic and chiral fashion as a spacer for bisdistamycin formation. Synthesis and DNA binding study. Tetrahedron 2006, 62, 8591–8600. [Google Scholar]
- Ghosh, S.; Usharani, D.; Paul, A.; De, S.; Jennis, E.D.; Bhattacharya, S. Design, synthesis, and DNA binding properties of photoisomerizable azobenzene-distamycin conjugates: an experimental and computational study. Bioconjugate Chem. 2008, 19, 2332–2345. [Google Scholar]
- Vázquez, O.; Blanco-Canosa, J.B.; Vázquez, M.E.; Martinez-Costas, J.; Castedo, L.; Mascareñas, J.L. Efficient DNA binding and nuclear uptake by distamycin derivatives conjugated to octa-arginine sequences. Chem. Biochem. 2008, 9, 2822–2829. [Google Scholar]
- Ghosh, S.; Defrancq, E.; Lhomme, J.H.; Dumy, P.; Bhattacharya, S. Efficient comjugation and characterization of distamycin-based peptides with selected oligonucleotide stretches. Bioconjugate Chem. 2004, 15, 520–529. [Google Scholar] [CrossRef]
- Randazzo, A.; Galeone, A.; Mayol, L. 1H-NMR study of the interaction of distamycin A and netropsin with the parallel stranded tetraplex [d(TGGGGT)]4. Chem. Commun. 2001, 11, 1030–1031. [Google Scholar]
- Cocco, M. J.; Hanakahi, L.A.; Huber, M. D.; Maizels, N. Specific interactions of distamycin with G-quadruplex DNA. Nucleic Acids Res. 2003, 31, 2944–2951. [Google Scholar]
- Moore, M.J.B.; Cuenca, F.; Searcey, M.; Neidle, S. Synthesis of distamycin A polyamides targeting G-qyadruplex DNA. Org. Biomol. Chem. 2006, 4, 3479–3488. [Google Scholar] [CrossRef]
- Bartulewicz, D.; Markowska, A.; Wołczyński, S.; Dąbrowska, M.; Różański, A. Molecular modelling, synthesis and antitumour activity of carbocyclic analogues of netropsin and distamycin – new carriers of alkylating elements. Acta Biochim. Polon 2000, 47, 23–35. [Google Scholar]
- Bielawski, K.; Bielawska, A.; Bartulewicz, D.; Różański, A. Molecular modelling of the interaction of carbocyclic analogues of netropsin and distamycin with d(CGCGAATTCGCG)2. Acta Biochim. Polon. 2000, 47, 855–866. [Google Scholar]
- Drozdowska, D.; Rusak, M.; Miltyk, W.; Midura-Nowaczek, K. Synthesis and biological evaluation of distamycin analogues - new potential anticancer agents. Arch. Pharm. Chem. Life Sci. 2009, 342, 87–93. [Google Scholar]
- Drozdowska, D.; Rusak, M.; Bielawski, T.; Midura-Nowaczek, K. Analogues of distamycin –– synthesis and biological evaluation of new aromatic oligopeptides, potential anticancer agents. Acta Polon. Pharm. 2009, 66, 633–638. [Google Scholar]
- Bielawski, K.; Bielawska, A.; Bartulewicz, D. Carbocyclic analogues of netropsin and distamycin: DNA-binding properties and inhibition of DNA topoisomerases. Arch. Pharm. Pharm. Med. Chem. 2001, 9, 422–426. [Google Scholar]
- Drozdowska, D.; Rusak, M.; Bielawski, T.; Midura-Nowaczek, K. Carbocyclic potential DNA minor groove binders and their biological evaluation. J. Enz. Inhib. Med. Chem. 2010, 25, 629–634. [Google Scholar] [CrossRef]
- Bartulewicz, D.; Bielawski, K.; Bielawska, A.; Różański, A. Synthesis, molecular modelling, and antiproliferative and cytotoxic effects of carbocyclic derivatives of distamycin with chlorambucil moiety. Eur. J. Med. Chem. 2001, 36, 461–467. [Google Scholar] [CrossRef]
- Mařík, J.; Song, A.; Lam, K.S. Detection of primary aromatic amines on solid phase. Tetrahedron Lett. 2003, 44, 4319–4320. [Google Scholar] [CrossRef]
- Bartulewicz, D.; Bielawski, K.; Markowska, A.; Zwierz, K.; Pućkowska, A.; Różański, A. Synthetic analogues of netropsin and distamycin – synthesis of a new pyridine and carbocyclic analogues of the pyrrolecarboxamide antitumour antibiotics. Acta Biochim. Polon. 1998, 45, 31–57. [Google Scholar]
- Kamiński, Z.J.; Kolesińska, B.; Kolesińska, J.; Sabatino, G.; Chelli, M.; Rovero, P.; Błaszczyk, M.; Główka, M.L.; Papini, A.M. N-Triazinylammonium tetrafluoroborates. A new generation of efficient coupling reagents useful for peptide synthesis. J. Am. Chem. Soc. 2005, 127, 16912–16920. [Google Scholar]
- Sample Availability: Samples of the compounds D1-D6 are available from the author.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Danuta, D. New Solid Phase Synthesis of Distamycin Analogues. Molecules 2011, 16, 3066-3076. https://doi.org/10.3390/molecules16043066
Danuta D. New Solid Phase Synthesis of Distamycin Analogues. Molecules. 2011; 16(4):3066-3076. https://doi.org/10.3390/molecules16043066
Chicago/Turabian StyleDanuta, Drozdowska. 2011. "New Solid Phase Synthesis of Distamycin Analogues" Molecules 16, no. 4: 3066-3076. https://doi.org/10.3390/molecules16043066