Kazinol Q from Broussonetia kazinoki Enhances Cell Death Induced by Cu(ll) through Increased Reactive Oxygen Species
Abstract
:1. Introduction
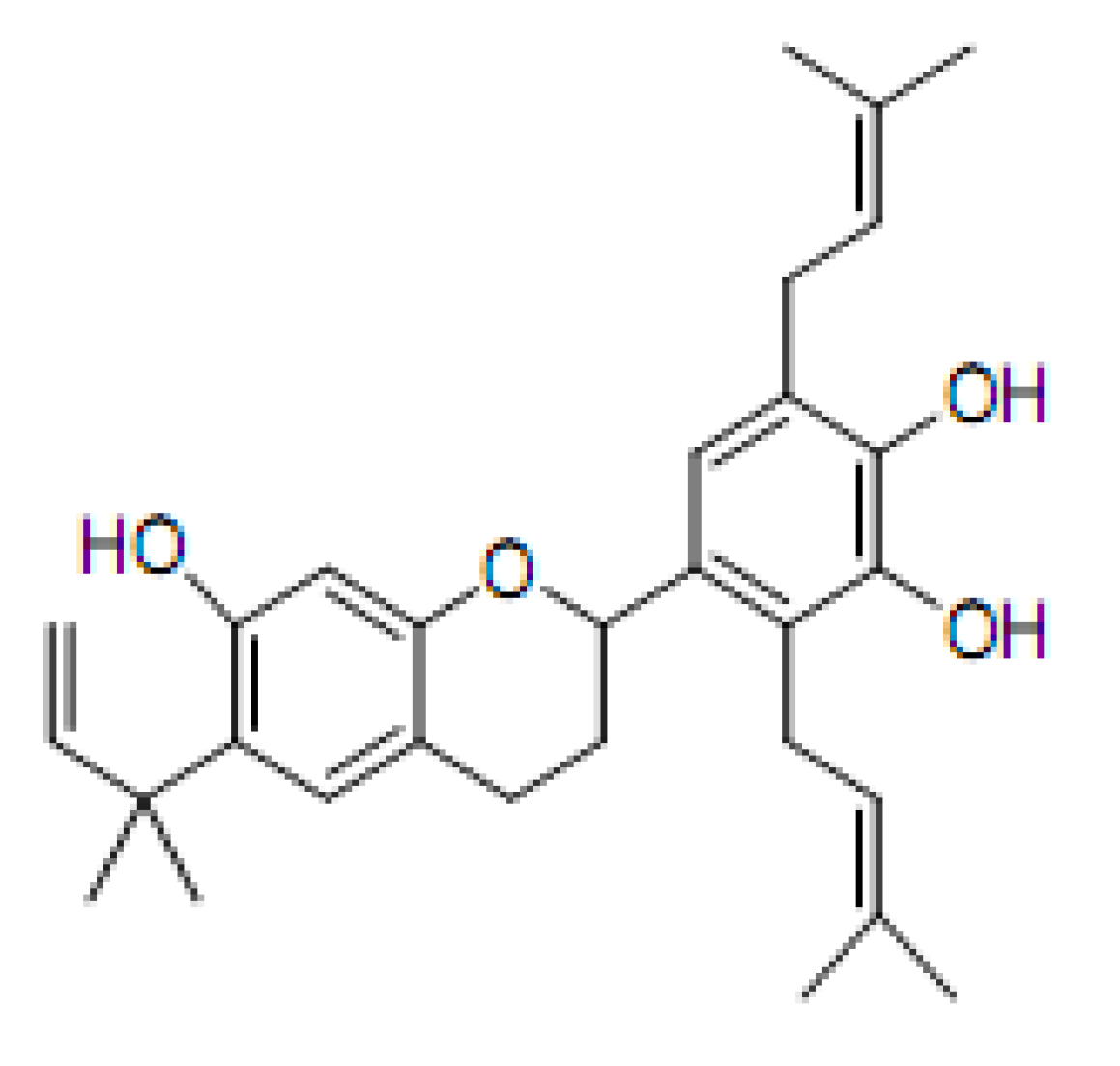
2. Results
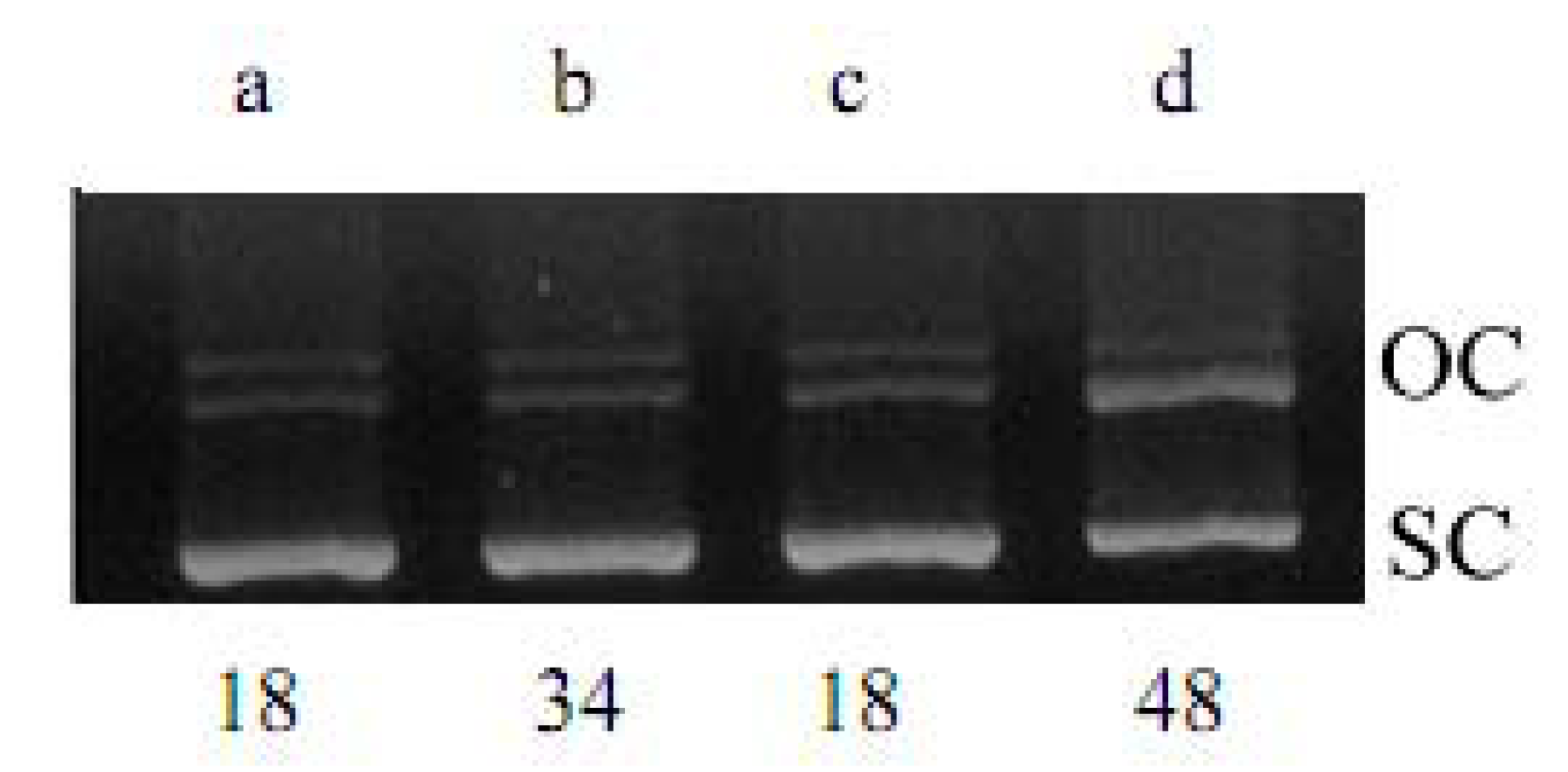
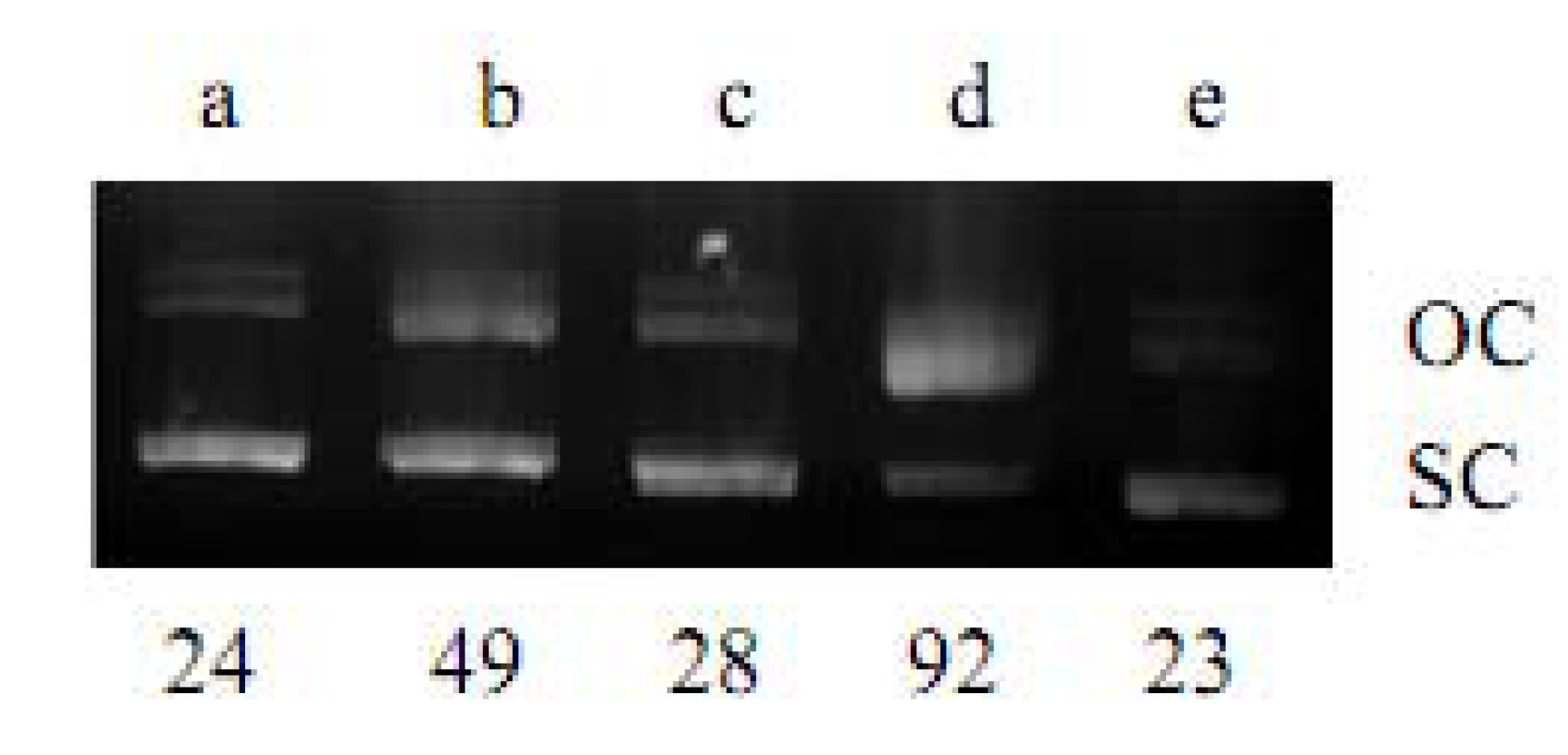
2.1. DNA Strand-Scission of Compound

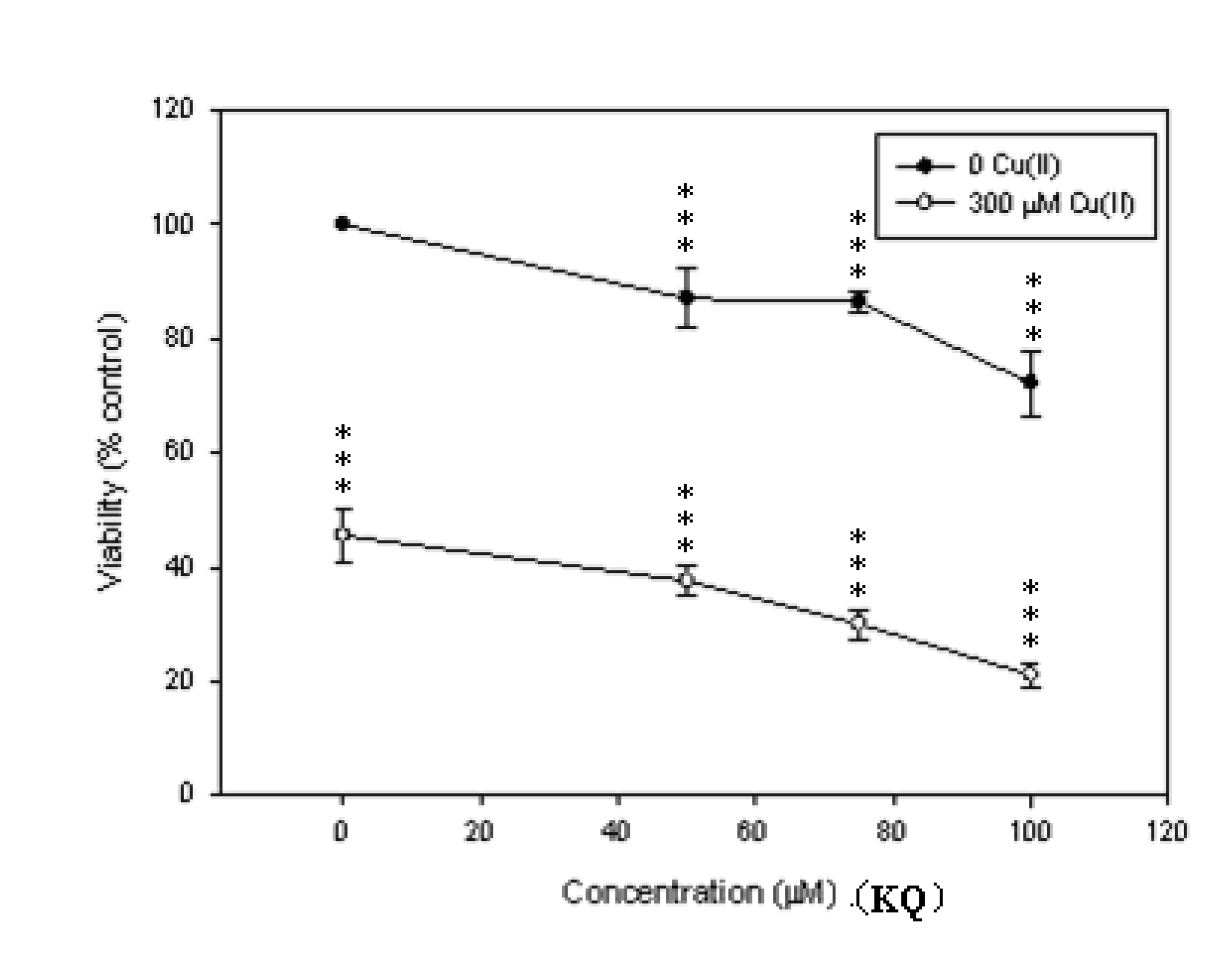
2.2. Cytotoxic Effect of KQ on SCM-1 Cells in the Absence or Presence of Cu(II)

2.3. Mitochondrial Membrane Potential and Cell Apoptosis and Necrosis

2.4. Compound KQ Increased ROS Production with and without Cu(ll) Measured by Flow Cytometry

3. Discussion
4. Experimental
4.1. Chemicals
4.2. DNA Strand-Scission Assay
4.3. Cell Culture and MTT Assay for Cell Viability/Proliferation
4.4. Measurement of Mitochondrial Membrane Potential Depolarization
4.5. Annexin V/PI Staining
4.6. Compound KQ Induced ROS Production with and without Cu(II) Measured by Flow Cytometry
4.7. Statistical Analysis
5. Conclusions
Acknowledgements
References
- Ma, J.; Jone, S.H.; Hecht, S.M. Phenolic acid amides: a new type of DNA strand scission agent from Piper caninum. Bioorg. Med. Chem. 2004, 12, 3885–3889. [Google Scholar] [CrossRef]
- Kuo, P.L.; Chan, C.Y.; Tzeng, T.F.; Lin, C.C.; Hsu, Y.L. Involvement of reactive oxygen spcis/c-Jun NH2-terminal kinase pathway in katomolide A induces apoptosis in human breast cancer cells. Toxicol. Appl. Pharmacol. 2008, 229, 215–226. [Google Scholar] [CrossRef]
- Hsu, H.Y.; Tsang, S.F.; Lin, K.W.; Yang, S.C.; Lin, C.N. Cell death induced by flavonoid glycosides with and without copper. Food Chem. Toxicol. 2008, 46, 2394–2401. [Google Scholar]
- Ko, H.H.; Yen, M.H.; Wu, R.R.; Won, S.J.; Lin, C.N. Cytotoxic isoprenylated flavans of Broussonetia kazinoki. J. Nat. Prod. 1999, 62, 164–166. [Google Scholar] [CrossRef]
- Ahsan, H.; Hadi, S.M. Strand scission in DNA induced by curcumin in the presence of Cu(II). Cancer Lett. 1998, 124, 23–30. [Google Scholar]
- Krumschnabel, G.; Manzl, C.; Berger, C.; Hofer, B. Oxidative stress, mitochondrial permeability transition, and cell death in Cu-exposed trout hepatocytes. Toxicol. Appl. Pharmacol. 2005, 209, 62–73. [Google Scholar] [CrossRef]
- Pourahmad, J.; Ross, S.; O'Brien, P.J. Lysosomal involvement in hepatocyte cytotoxicity induced by Cu2+ but Cd2+. Free Radic. Biol. 2001, 30, 89–97. [Google Scholar] [CrossRef]
- Hancock, J.T.; Desikan, R.; Neill, S.J. Role of reactive oxygen species in cell signaling pathways. Biochem. Soc. Trans. 2001, 29, 345–350. [Google Scholar] [CrossRef]
- Chandhuri, S.K.; Huang, L.; Fullas, F.; Brown, D.M.; Wani, M.C.; Wall, M.E. Isolation and structure identification of an active DNA strand-scission agent, (+)-3,4-dihydroxy-8,9-methylenedioxypterocarpan. J. Nat. Prod. 1995, 58, 1966–1969. [Google Scholar] [CrossRef]
- Sample Availability: Sample of the compound is available from the corresponding author.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wei, B.-L.; Chen, Y.-C.; Hsu, H.-Y. Kazinol Q from Broussonetia kazinoki Enhances Cell Death Induced by Cu(ll) through Increased Reactive Oxygen Species. Molecules 2011, 16, 3212-3221. https://doi.org/10.3390/molecules16043212
Wei B-L, Chen Y-C, Hsu H-Y. Kazinol Q from Broussonetia kazinoki Enhances Cell Death Induced by Cu(ll) through Increased Reactive Oxygen Species. Molecules. 2011; 16(4):3212-3221. https://doi.org/10.3390/molecules16043212
Chicago/Turabian StyleWei, Bai-Luh, Ying-Chieh Chen, and Hsue-Yin Hsu. 2011. "Kazinol Q from Broussonetia kazinoki Enhances Cell Death Induced by Cu(ll) through Increased Reactive Oxygen Species" Molecules 16, no. 4: 3212-3221. https://doi.org/10.3390/molecules16043212



