Inhibition of Inflammatory Mediators by Neobavaisoflavone in Activated RAW264.7 Macrophages
Abstract
:1. Introduction

2. Results and Discussion

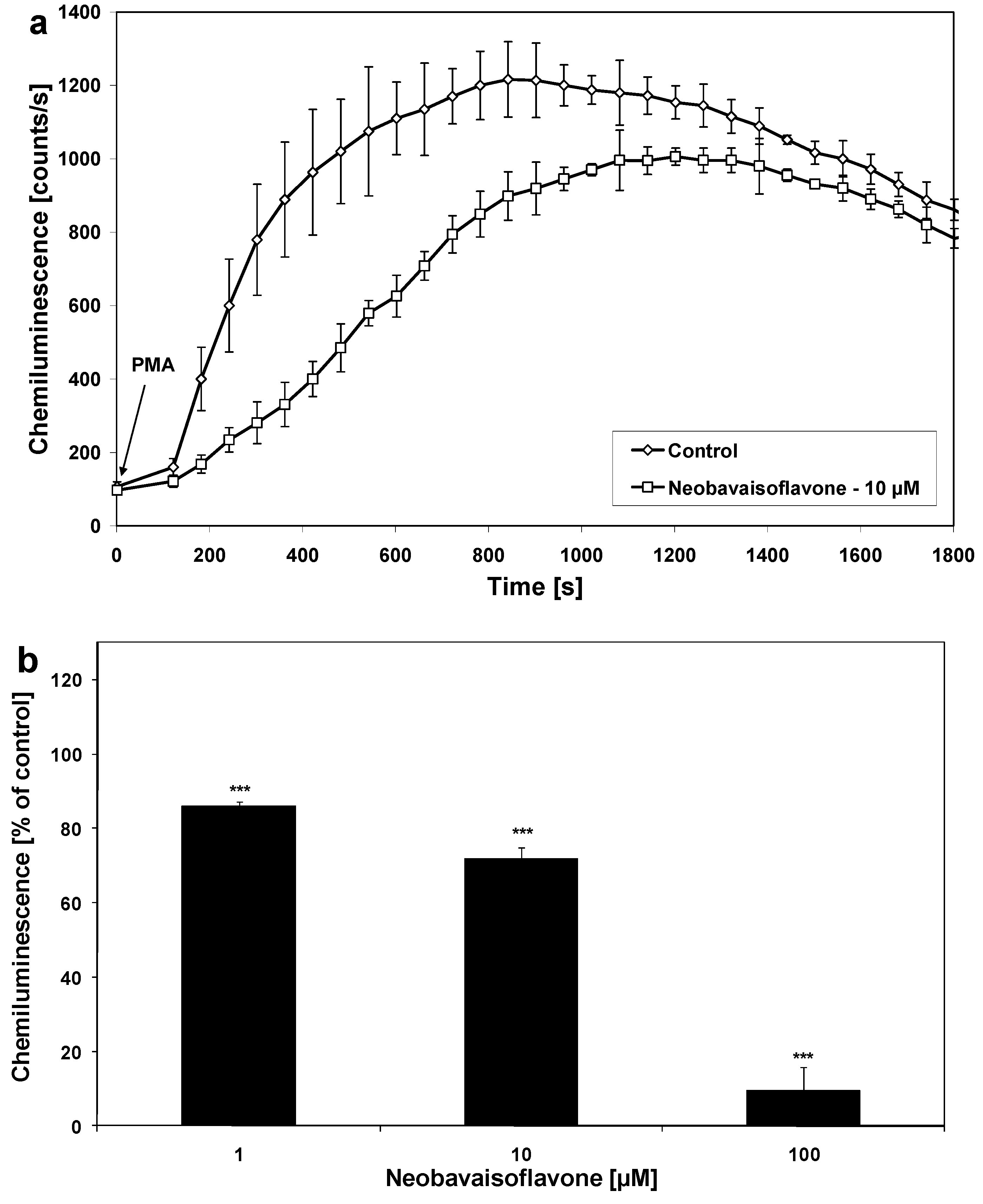
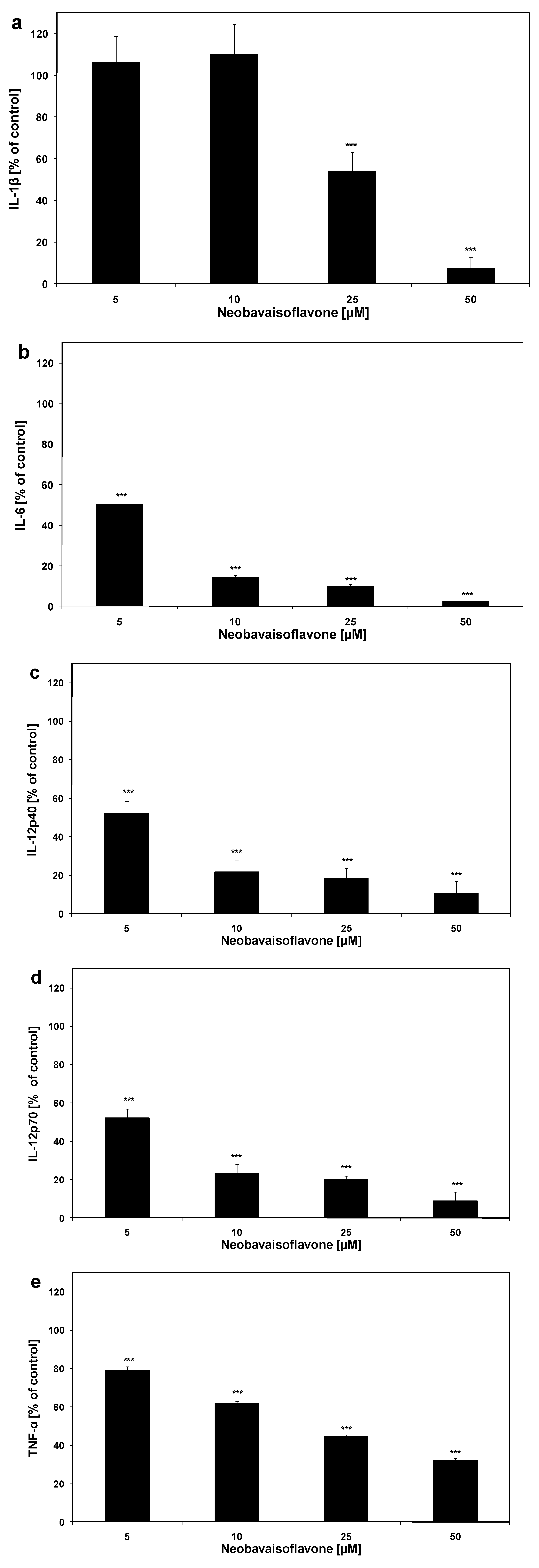
| Inflammatory mediator | ED50 [μM] |
|---|---|
| NO (nitrite) | 25.0 |
| IL-1β | 23.11 |
| IL-6 | 5.03 |
| IL-12p40 | 5.23 |
| IL-12p70 | 5.26 |
| TNF-α | 18.80 |
3. Experimental
3.1. General
3.2. Cell Culture
3.3. Cell Viability Assay
3.4. Lactate Dehydrogenase Release Assay
3.5. Quantification of Nitric oxide Production
3.6. Detection of ROS and RNS Production by Chemiluminescence
3.7. Multiplex Bead-Based Cytokine Assay
3.8. Statistical Analysis
4. Conclusions
Acknowledgements
References and Notes
- Blonska, M.; Czuba, Z.P.; Krol, W. Effect of flavone derivatives on interleukin-1beta (IL-1beta) mRNA expression and IL-1beta protein synthesis in stimulated RAW 264.7 macrophages. Scan. J. Immunol. 2003, 52, 162–166. [Google Scholar]
- Zhang, L.; Cheng, Y.; Liu, A.; Wang, H.; Wang, Y.; Du, G. Antioxidant, anti-inflammatory and anti-influenza properties of compounds from Chaenomeles speciosa. Molecules 2010, 15, 8507–8517. [Google Scholar] [CrossRef]
- Guimaraes, R.; Barros, L.; Barreira, J.C.; Sousa, M.L.; Carvalho, A.M.; Ferreira, I.C. Targeting excessive free radicals with peels and juices of citrus fruits: grapefruit, lemon, lime and orange. Food Chem. Toxicol. 2010, 48, 99–106. [Google Scholar] [CrossRef]
- Bronikowska, J.; Szliszka, E.; Czuba, Z.P.; Zwolinski, D.; Szmydki, B.; Krol, W. The combination of TRAIL and isoflavones enhances apoptosis in cancer cells. Molecules 2010, 15, 2000–2015. [Google Scholar] [CrossRef]
- Szliszka, E.; Bronikowska, J.; Czuba, Z.P.; Krol, W. Isoflavones augment the effect of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) on prostate cancer cells. CEJ Urol. 2010, 63, 182–186. [Google Scholar]
- Szliszka, E.; Czuba, Z.P.; Sędek, L.; Paradysz, A.; Król, W. Enhanced TRAIL-mediated apoptosis in prostate cancer cells by the bioactive compounds neobavaisoflavone and psoralidin isolated from Psoralea corylifolia. Pharmacol. Rep. 2011, 63, 139–148. [Google Scholar]
- Bhalla, V.K.; Ramdas, N.U.; Sukh, D. Some new Flavonoids from Psoralea corylifolia. Tetrahedron. Lett. 1968, 9, 2401–2406. [Google Scholar] [CrossRef]
- Bajwa, B.S.; Khanna, P.L.; Seshadri, T.R. Components of different parts of seeds (fruits) of Psoralea corylifolia. Indian J. Chem. 1974, 12, 15–19. [Google Scholar]
- Latha, P.G.; Evans, D.A.; Panikkar, K.R.; Jayavardhanan, K.K. Immunomodulatory and antitumour properties of Psoralea corylifolia seeds. Fitoterapia 2000, 71, 223–31. [Google Scholar] [CrossRef]
- Majinda, R.R.T.; Wanjala, C.C.W.; Juma, B.F. Bioactive non-alkaloidal constituents from the genus Erythrina. In Studies in Natural Products Chemistry - Bioactive Natural Products (Part L); Atta-ur-Rahman, Ed.; Elsevier B.V: Amsterdam, The Netherlands, 2005; Volume 32, pp. 821–853. [Google Scholar]
- Chacha, M.; Bojase-Moleta, G.; Majinda, R.R.T. Antimicrobial and radical scavenging flavonoids from the stem wood of Erythrina latissima. Phytochemistry 2005, 66, 99–104. [Google Scholar] [CrossRef]
- Khushboo, P.S.; Jadhav, V.M.; Kadam, V.J.; Sathe, N.S. Psoralea corylifolia Linn.-“Kushtanashini”. Pharmacogn. Rev. 2010, 4, 69–76. [Google Scholar] [CrossRef]
- Prasad, N.R.; Anandi, C.; Balasubramanian, S.; Pugalendi, K.V. Antidermatophytic activity of extracts from Psoralea corylifolia (Fabaceae) correlated with the presence of a flavonoid compound. J. Ethnopharmacol. 2004, 91, 21–24. [Google Scholar] [CrossRef]
- Xiao, D.; Li, G.; Chen, L.; Zhang, Z.; Yin, J.; Wu, T.; Cheng, Z.; Wei, X.; Wang, Z. Isolation of antioxidants from Psoralea corylifolia fruits using high-speed counter-current chromatography guided by thin layer chromatography-antioxidant autographic assay. J. Chromatogr. A 2010, 1217, 5470–5476. [Google Scholar] [CrossRef]
- Zhao, L.; Wu, M.; Xiang, B. Analysis of Psoralea corylifolia L fruits in different regions. Chem. Pharm. Bull. 2005, 53, 1054–1057. [Google Scholar] [CrossRef]
- Lee, M.H.; Kim, J.Y.; Ryu, J. Prenylflavones from Psoralea corylifolia inhibit nitric oxide synthase expression through the inhibition of I-κB-α degradation in activated microglial cells. Biol. Pharm. Bull. 2005, 28, 2253–2257. [Google Scholar] [CrossRef]
- Matsuda, H.; Kiyohara, S.; Sugimoto, S.; Ando, S.; Nakamura, S.; Yoshikawa, M. Bioactive constituents from Chinese natural medicines. XXXIII. Inhibitors from the seeds of Psoralea corylifolia on production of nitric oxide in lipopolysaccharide-activated macrophages. Biol. Pharm. Bull. 2009, 32, 147–149. [Google Scholar] [CrossRef]
- Wang, Y.; Hong, C.; Zhou, C.; Xu, D.; Qu, H.; Cheng, Y. Screening antitumor compounds psoralen and isopsoralen from Psoralea corylifolia L seeds. Evid. Based Complement. Altern. Med. 2009, 8, 1–7. [Google Scholar]
- Zhao, L.; Huang, C.Y.; Shan, Z.; Xiang, B.G.; Mei, L.H. Fingerprint analysis of Psoralea corylifolia by HLPC and LC-MS. J. Chromatogr. B 2005, 821, 67–74. [Google Scholar] [CrossRef]
- Ruan, B.; Kong, L.Y.; Takaya, Y.; Niwa, M. Studies on the chemical constituents of Psoralea corylifolia L. J. Asian Nat. Prod. Res. 2007, 9, 41–44. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, B.; Yang, J.; Hu, L.; Su, Y.; Gao, X. A rapid method for the analysis of ten compounds in Psoralea corylifolia L. by UPLC. Chromatographia 2009, 70, 199–204. [Google Scholar] [CrossRef]
- Kim, A.R.; Cho, J.Y.; Zou, Y.; Choi, J.S.; Chung, H.Y. Flavonoids differentially modulate nitric oxide production pathways in lipopolysaccharide-activated RAW264.7 cells. Arch. Pharm. Res. 2005, 28, 297–304. [Google Scholar] [CrossRef]
- Yoon, S.; Lee, Y.; Park, S.K.; Kim, H.; Bae, H.; Kim, H.M.; Ko, S.; Choi, H.Y.; Oh, M.S.; Park, W. Anti-inflammatory effects of Scutellaria baicalensis water extract on LPS-activated RAW264.7 macrophages. J. Ethnopharmacol. 2009, 125, 286–290. [Google Scholar] [CrossRef]
- Krenn, L.; Paper, D.H. Inhibition of angiogenesis and inflammation by an extract of red clover (Trifolium pretense L.). Phytomedicine 2009, 16, 1083–1088. [Google Scholar] [CrossRef]
- Król, W.; Czuba, Z.P.; Threadgill, M.D.; Cunningham, B.D.; Pietsz, G. Inhibition of nitric oxide (NO) production in murine macrophages by flavones. Biochem. Pharmacol. 1995, 50, 1031–1035. [Google Scholar] [CrossRef]
- Kole, L.; Giri, B.; Manna, S.K.; Pal, B.; Ghosh, S. Biochanin-A, an isoflavon, showed anti-proliferative and anti-inflammatory activities through the inhibition of iNOS expression, p38-MAPK and ATF-2 phosphorylation and blocking NFκB nuclear translocation. Eur. J. Pharmacol. 2011, 653, 8–15. [Google Scholar] [CrossRef]
- Krol, W.; Shani, J.; Czuba, Z.P.; Scheller, S. Modulating luminol-dependent chemiluminescence of neutrophils by flavones. Z. Naturforsch. C 1992, 47, 889–892. [Google Scholar]
- Król, W.; Czuba, Z.P.; Scheller, S.; Paradowski, Z.; Shani, J. Structure-activity relationship in the ability of flavonols to inhibit chemiluminescence. J. Ethnopharmacol. 1994, 41, 121–126. [Google Scholar] [CrossRef]
- Ishihara, K.; Hirano, T. IL-6 in autoimmune disease and chronic inflammatory proliferative disease. Cytokine Growth Factor Rev. 2002, 13, 357–368. [Google Scholar] [CrossRef]
- Jana, M.; Dasgupta, S.; Saha, R.N.; Liu, X.; Pahan, K. Induction of tumor necrosis factor-alpha (TNF-alpha) by interleukin-12 p40 monomer and homodimer in microglia and macrophages. J. Neurochem. 2003, 86, 519–528. [Google Scholar]
- Hu, X.D.; Yang, Y.; Zhong, X.G.; Zhang, X.H.; Zhang, Y.N.; Zheng, Z.P.; Zhou, Y.; Tang, W.; Yang, Y.F.; Hu, L.H.; Zuo, J.P. Anti-inflammatory effects of Z23 on LPS-induced inflammatory responses in RAW264.7 macrophages. J. Ethnopharmacol. 2008, 120, 447–451. [Google Scholar] [CrossRef]
- Szliszka, E.; Czuba, Z.P.; Jernas, K.; Krol, W. Dietary flavonoids sensitize HeLa cells to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). Int. J. Mol. Sci. 2008, 9, 56–64. [Google Scholar] [CrossRef]
- Szliszka, E.; Czuba, Z.P.; Domino, M.; Mazur, B.; Zydowicz, G.; Krol, W. Ethanolic extract of propolis (EEP) enhances the apoptosis-inducing potential of TRAIL in cancer cells. Molecules 2009, 14, 738–754. [Google Scholar] [CrossRef]
- Szliszka, E.; Czuba, Z.P.; Mazur, B.; Sedek, L.; Paradysz, A.; Krol, W. Chalcones enhance TRAIL-induced apoptosis in prostate cancer cells. Int. J. Mol. Sci. 2010, 11, 1–13. [Google Scholar]
- Szliszka, E.; Czuba, Z.P.; Mazur, B.; Paradysz, A.; Krol, W. Chalcones and dihydrochalcones augment TRAIL-mediated apoptosis in prostate cancer cells. Molecules 2010, 15, 5336–5353. [Google Scholar] [CrossRef]
- Baxter, M.A.; Leslie, R.G.; Reeves, W.G. The stimulation of superoxide anion production in guinea-pig peritoneal macrophages and neutrophils by phorbol myristate acetate, opsonized zymosan and IgG2-containing soluble immune complexes. Immunology 1983, 48, 657–665. [Google Scholar]
- Sample Availability: Samples of neobavaisoflavone are available from Alexis Biochemicals (San Diego, CA, USA).
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Szliszka, E.; Skaba, D.; Czuba, Z.P.; Krol, W. Inhibition of Inflammatory Mediators by Neobavaisoflavone in Activated RAW264.7 Macrophages. Molecules 2011, 16, 3701-3712. https://doi.org/10.3390/molecules16053701
Szliszka E, Skaba D, Czuba ZP, Krol W. Inhibition of Inflammatory Mediators by Neobavaisoflavone in Activated RAW264.7 Macrophages. Molecules. 2011; 16(5):3701-3712. https://doi.org/10.3390/molecules16053701
Chicago/Turabian StyleSzliszka, Ewelina, Dariusz Skaba, Zenon P. Czuba, and Wojciech Krol. 2011. "Inhibition of Inflammatory Mediators by Neobavaisoflavone in Activated RAW264.7 Macrophages" Molecules 16, no. 5: 3701-3712. https://doi.org/10.3390/molecules16053701




