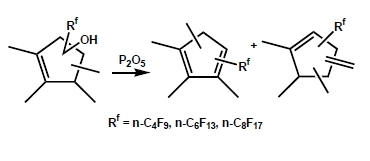
Dehydration of (Perfluoroalkyl)tetramethylcyclopentenols
Abstract
:1. Introduction
2. Results and Discussion
2.1. Screening of Dehydration Agents
| Reagent(s) | Solvent | Temperature [°C] /Time [h] | Sum of unreacted alcohols [%] | Sum of dienes [%] |
|---|---|---|---|---|
| POCl3 | pyridine | 90/4 | 10 | 59 |
| BF3·Et2O | diethyl ether | 35/9 | 14 | 77 b |
| SOCl2 | benzene | 90/9 | 2 | 91 c |
| PCl5/pyridine | benzene | 90/10 | 0 | 90 c |
| P2O5 | benzene | 90/9 | 6 | 89 |
| P2O5 | toluene | 120/7 a | 5 | 86 |
| P2O5 / BF3·Et2O | diethyl ether | 42/8 | 14 | 83 |
| P2O5 / BF3·Et2O | diethyl ether | 25/168 | 10 | 86 |
2.1. Kinetics of Dehydration of (Perfluoroalkyl)tetramethylcyclopentenols with P2O5
2.2.1. Identification of Products


| Isomer | Type | tRet [s] | Molecular ion M+. (m/z) | Base peak (m/z) | Other ions (m/z) | Molar fraction at t = x min | |
|---|---|---|---|---|---|---|---|
| 0 | 360 | ||||||
| A4 | diene | 513 | 340 | 121 | 325, 305, 171, 106, 91, 69 | 0.009 | 0.076 |
| C4 | diene | 532 | 340 | 121 | 105, 93, 69 | 0 | 0.016 |
| D4 | diene | 544 | 340 | 325 | 305, 171, 121, 91, 69 | 0.001 | 0.024 |
| E4 | diene | 560 | 340 | 171 | 320, 305, 156, 91, 69 | 0.004 | 0.105 |
| F4 | diene | 568 | 340 | 171 | 320, 305, 151, 91, 69 | 0 | 0.122 |
| G4 | diene | 575 | 340 | 171 | 320, 305, 151, 91, 69 | 0.024 | 0.607 |
| H4 | diene | 587 | 320 | 305, 201, 171, 151, 69 | 0 | 0.015 | |
| I4 | diene | 603 | 343 | 343 | 305, 171, 121, 91, 69 | 0 | 0.005 |
| V4 | alcohol | 608 | 139 | 341,171, 121, 69 | 0.813 | 0.001 | |
| W4 | alcohol | 611 | 139 | 340,171, 121, 69 | 0.016 | 0 | |
| X4 | alcohol | 618 | 121 | 139, 95, 69 | 0.128 | 0.032 | |
| Isomer | Type | tRet [s] | Mol. ion M+. (m/z) | Base peak (m/z) | Other ions (m/z) | Molar fraction at t = x min | |||
|---|---|---|---|---|---|---|---|---|---|
| 0b | 483 b | 0 c | 480 c | ||||||
| A6 | diene | 577 | 440 | 121 | 425, 171, 91, 69 | 0.011 | 0.119 | 0.025 | 0 |
| B6 | diene | 581 | 113 | 403, 69, 57 | 0.015 | 0.019 | 0.025 | 0.054 | |
| C6 | diene | 591 | 440 | 69 | 121, 93 | 0.004 | 0.013 | 0.002 | 0.002 |
| D6 | diene | 599 | 440 | 69 | 425, 171, 121, 91 | 0.002 | 0.018 | 0.004 | 0.007 |
| E6 | diene | 609 | 440 | 171 | 420, 119, 69 | 0.005 | 0.105 | 0.012 | 0.031 |
| (F6)d | diene | 617 | 440 | 171 | 425, 151, 105, 69 | ||||
| G6 | diene | 620 | 440 | 171 | 420, 201, 151, 105, 69 | 0.048e | 0.679e | 0.108e | 0.252e |
| H6 | diene | 625 | 405 | 420, 186, 171, 151, 69 | 0.008 | 0.016 | 0 | 0.014 | |
| I6 | diene | 628 | 69 | 420, 401, 201, 181, 151, 119 | 0 | 0 | 0.006 | 0.536 | |
| J6 | diene | 636 | 420 | 201, 171, 151, 69 | 0 | 0 | 0.044 | 0.046 | |
| K6 | diene | 641 | 443 | 443 | 171, 121, 83, 69 | 0 | 0 | 0.005 | 0.005 |
| V6 | alcohol | 646 | 139 | 440,171, 121, 69 | 0.818 | 0.031 | 0.582 | 0 | |
| W6 | alcohol | 648 | 139 | 440,171, 121, 69 | 0.09 | 0.026 | 0.11 | 0 | |
| X6 | alcohol | 655 | 139 | 443,171, 121, 95, 69 | 0 | 0.005 | 0.079 | 0.051 | |
| Y6 | alcohol | 658 | 139 | 440,121, 95, 69 | 0.005 | 0.005 | 0 | 0 | |
| Isomer | Type | tRet [s] | Molecular ion M+. (m/z) | Base peak (m/z) | Other ions (m/z) |
|---|---|---|---|---|---|
| A8 | diene | 641 | 540 | 121 | 525,171, 105, 91, 69 |
| B8 | diene | 644 | 113 | 121, 69, 57 | |
| C8 | diene | 651 | 540 | 121 | 105, 91, 69 |
| D8 | diene | 657 | 540 | 121 | 525, 171, 105, 69 |
| F8 | diene | 666 | 540 | 171 | 521, 156, 121, 105, 69 |
| G8 | diene | 673 | 540 | 171 | 520, 201, 151, 69 |
| H8 | diene | 679 | 540 | 201 | 520, 501, 171, 151, 69 |
| V8 | alcohol | 694 | 139 | 540,121, 69 | |
| W8 | alcohol | 696 | 139 | 540,121, 69 | |
| X8 | alcohol | 702 | 139 | 540,121, 105, 95, 69 | |
| Y8 | alcohol | 706 | 139 | 540,121, 95, 69 | |
| Z8 | alcohol | 710 | 139 | 540,157, 113, 95, 69,56 |
2.2.2. Kinetic Observations


| Substrate | Solvent | Temp. (°C) | Rate constanta | Reaction order | Standard deviation | Time50 %c [min] | Time90 %c [min] |
|---|---|---|---|---|---|---|---|
| CPT-C6F13-OH | benzene | 80 | |||||
| total reaction | 0.022 | 2 | 0.042 | 36 | 385 | ||
| individual reaction: V6 to F6+G6+E6 | 0.036 | 2 | 0.032 | ||||
| CPT-C6F13-OH | Cl-benzene | 80 | |||||
| total reaction | 0.016 | 0 | 0.085 | 15 | 38 | ||
| individual reaction: V6 to F6+G6+E6 | 0.023 | 0b | 0.023 | ||||
| CPT-C4F9-OH | benzene | 80 | |||||
| total reaction | 0.071 | 2 | 0.017 | 14 | 114 | ||
| individual reaction: V4+X4 to F4+G4 | 0.056 | 2 | 0.086 |

3. Experimental
3.1. General
3.2. Screening Dehydration Experiments-General Procedure
3.3. Dehydration with POCl3
3.4. Dehydration with BF3·Et2O
3.5. Dehydration with SOCl2
3.6. Dehydration with PCl5
3.7. Dehydration with P2O5 in Benzene
3.8. Dehydration with P2O5 in Toluene (Microwave Heating)
3.9. Dehydration with P2O5 in the Presence of BF3·Et2O at 40 °C
3.10. Dehydration with P2O5 in the Presence of BF3·Et2O at Room Temperature
3.11. Kinetics
3.12. NMR Characterization of Identified Main Products
Isomer 9
Isomer 10
Isomers 12/13
4. Conclusions
Acknowledgements
References and Notes
- Handbook of Fluorous Chemistry; Gladysz, J.A.; Curran, D.P.; Horváth, I.T. (Eds.) Wiley-VCH: Weinheim, Germany, 2004.
- Horváth, I.T.; Rábai, J. Facile catalyst separation without water: Fluorous biphase hydroformylation of olefins. Science 1994, 266, 72–75. [Google Scholar]
- Horváth, I.T. Fluorous biphase chemistry. Acc. Chem. Res. 1998, 31, 641–650. [Google Scholar] [CrossRef]
- Betzemeier, B.; Knochel, P. Perfluorinated solvents – a novel reaction medium in organic chemistry. Top. Curr. Chem. 1999, 206, 61–78. [Google Scholar]
- Barthel-Rosa, L.P.; Gladysz, J.A. Chemistry in fluorous media: A user’s guide to practical considerations in the application of fluorous catalysis and reagents. Coord. Chem. Rev. 1999, 190, 587–605. [Google Scholar] [CrossRef]
- Vincent, J.-M.; Rabion, A.; Yachandra, V.K.; Fish, R.H. Fluorous biphasic catalysis: Complexation of 1,4,7-[C8F17(CH2)3]3-1,4,7-triazacyclononane with [M(C8F17(CH2)2CO2)2] (M = Mn, Co to provide perfluoroheprane-soluble catalysts for alkane and alkene functionalization in the presence of t-BuOOH and O2. Angew. Chem. Int. Ed. 1997, 36, 2346–2349. [Google Scholar] [CrossRef]
- Pozzi, G.; Mihali, V.; Foschi, F.; Penso, M.; Quici, S.; Fish, R.H. 3,5-Bis(n-perfluorooctyl)benzyltriethylammonium bromide (F-TEBA): An efficient, easily recoverable fluorous catalyst for solid-liquid PTC reactions. Adv. Synth. Catal. 2009, 351, 3072–3076. [Google Scholar] [CrossRef]
- Pozzi, G.; Shepperson, I. Fluorous chiral ligands for novel catalytic systems. Coord. Chem. Rev. 2003, 242, 115–124. [Google Scholar] [CrossRef]
- Hughes, R.P.; Trujillo, H.A. Selective solubility of organometallic complexes in saturated fluorocarbons. Synthesis of cyclopentadienyl ligands with fluorinated ponytails. Organometallics 1996, 15, 286–294. [Google Scholar] [CrossRef]
- Bříza, T.; Kvíčala, J.; Mysík, P.; Paleta, O. 2-(Perfluoroalkyl)ethyl triflates, building blocks for the synthesis of bis(polyfluoroalkylated) cyclopentadienes. Synlett 2001, 2001, 685–687. [Google Scholar]
- Bříza, T.; Kvíčala, J.; Paleta, O.; Čermák, J. Preparation of bis(polyfluoroalkyl)cyclopentadienes, new highly fluorophilic ligands for fluorous biphase catalysis. Tetrahedron 2002, 58, 3841–3846. [Google Scholar] [CrossRef]
- Kvíčala, J.; Bříza, T.; Paleta, O.; Auerová, K.; Čermák, J. Synthesis, Fluorophilicities and regioisomer composition of ferrocenes and rhodium complexes based on bis(polyfluoroalkylated) cyclopentadienes. Tetrahedron 2002, 58, 3847–3854. [Google Scholar] [CrossRef]
- Dinh, L.V.; Gladysz, J.A. Convenient syntheses of “heavy fluorous” cyclopentadienes and cyclopentadienyl complexes with three to five ponytails. Chem. Commun. 2004, 998–999. [Google Scholar]
- Dinh, L.V.; Gladysz, J.A. “Heavy fluorus” cyclopentadienes and cyclopentadienyl complexes with three to five ponytails: Facile syntheses from polybromocyclopentadienyl complexes, phase properties, and electronic effects. Chem. Eur. J. 2005, 11, 7211–7222. [Google Scholar] [CrossRef]
- Čermák, J.; Šťastná, L.; Sýkora, J.; Císařová, I.; Kvíčala, J. Trimethylsilylcyclopentadienes with polyfluorinated ponytails and mono- and bis(η5-cyclopentadienyl) titanium(IV) complexes derived from them. Organometallics 2004, 23, 2850–2854. [Google Scholar] [CrossRef]
- Merle, P.G.; Chéron, V.; Hagen, H.; Lutz, M.; Spek, A.L.; Deelman, B.-J.; van Koten, G. Fluorous zirconocene(IV) complexes and their olefin polymerization activity in toluene and fluorous biphasic solvent systems. Organometallics 2005, 24, 1620–1630. [Google Scholar] [CrossRef]
- Červenková Šťastná, L.; Auerová, K.; Kvíčala, J.; Čermák, J. Fluorophilic properties of (perfluorooctyl)ethyldimethylsilyl substituted and tetramethyl(perfluoroalkyl) substituted cyclopentadienes and their Ti(IV), Rh(III), and Rh(I) complexes. J. Organomet. Chem. 2007, 692, 1974–1982. [Google Scholar] [CrossRef]
- Červenková Šťastná, L.; Čermák, J.; Cuřínová, P.; Sýkora, J. Synthesis and fluxional behavior of new "heavy fluorous" cyclopentadienes. J. Organomet. Chem. 2010, 695, 537–545. [Google Scholar] [CrossRef]
- ermák, J.; Auerová, K.; Nguyen, H.T.T.; Blechta, V.; Vojtíšek, P.; Kvíčala, J. Synthesis of rhodium complexes with novel perfluoroalkyl substituted cyclopentadienyl ligands. Collect. Czech. Chem. Commun. 2001, 66, 382–396. [Google Scholar] [CrossRef]
- Čermák, J.; Žádný, J.; Krupková, A.; Lopatová, K.; Vlachová, A.; Nguyen Thi, T.H.; Šauliová, J.; Sýkora, J.; Císařová, I. Tetramethyl(perfluoroalkyl)cyclopentadienyl rhodium(III) complexes containing phosphorus and nitrogen monodentate donors. Crystal structure of [(η5-C5Me4C4F9)Rh(PPri3)Cl2. J. Organomet. Chem. 2007, 692, 1557–1570. [Google Scholar]
- Čermák, J.; Krupková, A.; Auerová, K.; Zamrzla, M.; Nguyen Thi, T.H.; Vojtíšek, P.; Císařová, I. Tetramethyl(perfluoroalkyl)cyclopentadienyl rhodium(I) complexes with ethylene or diene ligands. Crystal structure of [(η5-C5Me4C6F13)Rh(CO)2]. J. Organomet. Chem. 2010, 695, 375–381. [Google Scholar]
- Gassman, P.G.; Mickelson, J.W.; Sowa, J.R., Jr. 1,2,3,4-Tetramethyl-5-(trifluoromethyl)cyclopentadiene: A unique ligand with the steric properties of pentamethylcyclopentadienide and the electronic properties of cyclopentadienide. J. Am. Chem. Soc. 1992, 114, 6942–6944. [Google Scholar] [CrossRef]
- Marken, F.; Marx, H.W.; Englert, U. Crystal-structure of twinned (η5-C5(CH3)4CF3)(η5-C5(CH3)5)Ru. Struct. Chem. 1994, 5, 177–181. [Google Scholar] [CrossRef]
- Gassman, P.G.; Sowa, J.R., Jr.; Hill, M.G.; Mann, K.R. Electrochemical studies of organometallic complexes with tetra-n-butylammonium tetrakis[3,5-bis(trifluoromethyl)phenyl]borate as the electrolyte. X-ray crystal structure of [C5(CF3)(CH3)4]Fe(C5H5). Organometallics 1995, 14, 4879–4885. [Google Scholar] [CrossRef]
- Baschky, M.C.; Sowa, J.R., Jr.; Gassman, P.G.; Kass, S.R. Gas-phase generation of trifluoromethyl cyclopentadienides. J. Chem. Soc. Perkin Trans. 2 1996, 213–215. [Google Scholar]
- Barthel-Rosa, L.P.; Sowa, J.R., Jr.; Gassman, P.G.; Fischer, J.; McCarty, B.M.; Goldsmith, S.L.; Gibson, M.T.; Nelson, J.H. Syntheses, properties, and X-ray crystal structures of iron and ruthenium compounds with the η5-C5Me4CF3 ligand. Compounds of the type [(η5-C5Me4CF3)M(µ-CO)(CO)]2 (M = Fe, Ru. Organometallics 1997, 16, 1595–1603. [Google Scholar] [CrossRef]
- Gusev, O.V.; Ievlev, M.A.; Lyssenko, K.A.; Petrovskii, P.V.; Ustynyuk, N.A.; Maitlis, P.M. Synthesis and properties of the dichloro[tetramethyl(trifluoromethyl)cyclopentadienyl]ruthenium dimer: X-ray structure of [M2(η5-C5Me4CF3)2Cl2(β-Cl)2] ( M = Ru, Rh). Inorg. Chim. Acta 1998, 280, 249–256. [Google Scholar] [CrossRef]
- Gusev, O.V.; Peganova, T.A.; Ievlev, M.A.; Kropotova, A.G.; Lyssenko, K.A.; Petrovskii, P.V.; Oprunenko, Y.F.; Ustynyuk, N.A. Synthesis of platinum complexes with η4-C5Me4(CF3)H ligand. X-ray structure of [Pt(η4-C5Me4H2)Cl2] and [Pt{η4-C5Me4(CF3)H}(η5-C5H5)]PF6. J. Organomet. Chem. 2001, 622, 221–227. [Google Scholar]
- Hughes, R.P.; Kowalski, A.S.; Lomprey, J.R. Preparation of the 1,2-di-tert-butylcyclopentadienyl anion and a transition metal derivative. Crystal structure of 1,1‘,2,2‘-tetra-tert-butylferrocene. Organometallics 1994, 13, 2691–2695. [Google Scholar] [CrossRef]
- Mellor, J.M.; El-Sagheer, A.H.; Salem, E.E.-D.M. A synthesis of trifluoromethyl-substituted naphthalenes. Tetrahedron Lett. 2000, 41, 7383–7386. [Google Scholar] [CrossRef]
- Mellor, J.M.; El-Sagheer, A.H.; El-Tamany, E.S.H.; Metwally, R.N. Synthesis of trifluoromethylnaphthalenes. Tetrahedron 2000, 56, 10067–10074. [Google Scholar] [CrossRef]
- Marquardt, D.W. An algorithm for least-squares estimation of nonlinear parameters. J. Soc. Industr. Appl. Math. 1963, 11, 431–441. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 9, 10 and 12/13 are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Čermák, J.; Nguyen Thi, T.H.; Včelák, J.; Krupková, A. Dehydration of (Perfluoroalkyl)tetramethylcyclopentenols. Molecules 2011, 16, 4031-4044. https://doi.org/10.3390/molecules16054031
Čermák J, Nguyen Thi TH, Včelák J, Krupková A. Dehydration of (Perfluoroalkyl)tetramethylcyclopentenols. Molecules. 2011; 16(5):4031-4044. https://doi.org/10.3390/molecules16054031
Chicago/Turabian StyleČermák, Jan, Thu Huong Nguyen Thi, Jaroslav Včelák, and Alena Krupková. 2011. "Dehydration of (Perfluoroalkyl)tetramethylcyclopentenols" Molecules 16, no. 5: 4031-4044. https://doi.org/10.3390/molecules16054031