Knoevenagel Reaction in [MMIm][MSO4]: Synthesis of Coumarins
Abstract
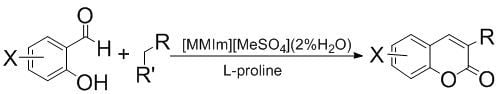
:1. Introduction
2. Results and Discussion
2.1. Knoevenagel Condensation of Benzaldehyde and Malononitrile

| Run a | Time[min] | Yield [%] b | % H2O |
|---|---|---|---|
| 1 | 1080 | 40 | - |
| 2 | 2 | 99 | 2 |
| 3 | 2 | 98 | 5 |
| 4 | 2 | 97 | 10 |
| 5 | 8 | 93 | 15 |
| 6 | 10 | 86 | 25 |
| 7 | 11 | 83 | 50 |
| 8 | 300 | 81 | 90 |
| Cycle a | Time[min] | Yield [%] b |
|---|---|---|
| 1 | 2 | 99 |
| 2 | 12 | 99 |
| 3 | 19 | 99 |
| 4 | 50 | 98 |
| 5 | 70 | 99 |
| 6 | 240 | 96 |
2.2. Knoevenagel Condensation with Different Substrates
2.3. Synthesis of Coumarins

| Entry | R | Time [min] | Yield [%] | Product | References |
|---|---|---|---|---|---|
| 1 | Cl | 5 | 99 | 2a | [9] |
| 2 | NMe2 | 7 | 98 | 2b | [23] |
| 3 | OMe | 3 | 92 | 2c | [9] |
| 4 | Me | 5 | 98 | 2d | [23] |

| Run | L-proline [equiv.] | T [°C] | Time [h] | Yield [%] |
|---|---|---|---|---|
| 1 | - | 90 | 18 | - |
| 2 | 0.2 | 90 | 4.5 | 96 |
| 3 | 0.5 | 90 | 1 | 90 |
| 4 | 1 | 90 | 0.5 | 98 |
| 5 | 1 | 70 | 2 | 90 |
| 6 | 1 | 50 | 6 | 80 |

| Entry | X | Y | R | R’ | Time [min] | Yield [%] b | References |
|---|---|---|---|---|---|---|---|
| 1 | H | H | CO2Me | CO2Me | 30 | 98 | [24] |
| 2 | H | H | COMe | CO2Me | 15 | 99 | [26,27] |
| 3 | H | H | CO2Et | CO2Et | 80 | 98 | [28,29] |
| 4 | H | H | COPh | CO2Et | 360 | 97 | [30] |
| 5 | 6-CH3 | H | CO2Me | CO2Me | 90 | 87 | - |
| 6 | 6-CH3 | H | CO2Et | CO2Et | 30 | 88 | [31] |
| 7 | 8-CH3 | H | CO2Me | CO2Me | 70 | 94 | - |
| 8 | 6-OMe | H | CO2Me | CO2Me | 15 | 87 | [32,33] |
| 9 | 7-OMe | H | CO2Me | CO2Me | 70 | 90 | [29] |
| 10 | 8-OMe | H | CO2Me | CO2Me | 30 | 94 | [32] |
| 11 | 6-Br | 8-OMe | CO2Me | CO2Me | 15 | 99 | - |
| 12 | 5,6-benzo | H | CO2Me | CO2Me | 90 | 99 | [34] |
| 13 | H | H | SO2Me | CO2Et | 90 | 96 | - |
| 14 | 7-OH | H | SO2Me | CO2Et | 480 | 99 | - |
| 15 | 7-Br | H | SO2Me | CO2Et | 360 | 93 | - |
| 16 | H | H | SO2Ph | CO2Me | 1440 | 92 | [35,36] |
| 17 | 7-OH | H | SO2Ph | CO2Me | 1140 | 99 | [35] |
| Run a | Time [h] | Yield [%] b |
|---|---|---|
| 1 | 0.5 | 98 |
| 2 | 1 | 98 |
| 3 | 3 | 98 |
| 4 | 5 | 98 |
| 5 | 6 | 98 |
3. Experimental
3.1. General
3.2. General Procedure for Knoevenagel Condensation of Benzaldehyde with Malononitrile
3.3. Recycling of Undried [MMIm][MSO4] (2.16% H2O) in the Knoevenagel Reaction of Benzaldehyde with Malononitrile
3.4. General Procedure for the Synthesis of Coumarins
3.5. Recycling of Undried [MMIm][MSO4] (2.16% H2O) + L-Proline in the the Synthesis of 3-(Methoxycarbonyl)coumarin (entry 1, Table 4)
4. Conclusions
Acknowledgments
References and Notes
- Freeman, F. Properties and reactions of ylidenemalononitriles. Chem. Rev. 1980, 80, 329–350. [Google Scholar] [CrossRef]
- Tietze, L.F. Domino reactions in organic synthesis. Chem. Rev. 1996, 96, 115–136. [Google Scholar] [CrossRef]
- Kraus, G.A.; Krolski, M.E. Synthesis of a precursor to quassimarin. J. Org. Chem. 1986, 51, 3347–3350. [Google Scholar] [CrossRef]
- Tietze, L.F.; Rackelmann, N. Domino reactions in the synthesis of heterocyclic natural products and analogs. Pure Appl. Chem. 2004, 76, 1967–1983. [Google Scholar] [CrossRef]
- Liang, F.-J.; Pu, Y.; Kurata, T.; Kido, J.; Nishide, H. Synthesis and electroluminescent property of poly(p-phenylenevinylene)s bearing triarylamine pendants. Polymer 2005, 46, 3767–3775. [Google Scholar] [CrossRef]
- Zahouily, M.; Salah, M.; Bahlaouane, B.; Rayadh, A.; Houmam, A.; Hamed, E.A.; Sebti, S. Solid catalysts for the production of fine chemicals: The use of natural phosphate alone and doped base catalysts for the synthesis of unsaturated arylsulfones. Tetrahedron 2004, 60, 1631–1635. [Google Scholar] [CrossRef]
- Poole, C.F. Chromatographic and spectroscopic methods for the determination of solvent properties of room temperature ionic liquids. J. Chromatog. A 2004, 1037, 49–82. [Google Scholar]
- Welton, T.; Wasserscheid, P. Ionic Liquids in Synthesis; Wiley-VCH: Weinheim, Germany, 2003; Volume 1, pp. 37–41. [Google Scholar]
- Zhang, J.; Jiang, T.; Han, B.; Zhu, A.; Ma, X. Knoevenagel condensation catalyzed by 1,1,3,3-tetramethylguanidium lactate. Syn. Commun. 2006, 36, 3305–3317. [Google Scholar] [CrossRef]
- Xin, X.; Guo, X.; Duan, H.; Lin, Y.; Sun, H. Efficient Knoevenagel condensation catalyzed by cyclic guanidinium lactate ionic liquid as medium. Catal. Commun. 2007, 8, 115–117. [Google Scholar] [CrossRef]
- Gao, G.; Lu, L.; Zou, T.; Gao, J.; Liu, Y.; He, M. Basic ionic liquid: A reusable catalyst for Knoevenagel Condensation in aqueous media. Chem. Res. Chinese U. 2007, 23, 169–172. [Google Scholar] [CrossRef]
- Yue, C.; Mao, A.; Wei, Y.; Lü, M. Knoevenagel condensation reaction catalyzed by task-specific ionic liquid under solvent-free conditions. Catal. Commun. 2008, 9, 1571–1574. [Google Scholar] [CrossRef]
- Wang, W.; Cheng, W; Shao, L.; Liu, C.; Yang, J. Henry and Knoevenagel reactions catalyzed by methoxyl propylamine acetate ionic liquid. Kinet. Catal. 2009, 50, 186–191. [Google Scholar] [CrossRef]
- Xu, D.-Z.; Liu, Y.; Shi, S.; Wang, Y. A simple, efficient and green procedure for Knoevenagel condensation catalyzed by [C4dabco][BF4] ionic liquid in water. Green Chem. 2010, 12, 514–517. [Google Scholar] [CrossRef]
- Santamarta, F.; Verdía, P.; Tojo, E. A simple, efficient and green procedure for Knoevenagel reaction in [MMIm][MSO4] ionic liquid. Catal. Commun. 2008, 9, 1779–1781. [Google Scholar]
- Gómez, E.; González, B.; Calvar, N.; Tojo, E.; Rodríguez, A. Physical properties of pure 1-ethyl-3-methylimidazolium ethylsulfate and its binary mixtures with ethanol and water at several temperatures. J. Chem. Eng. Data 2006, 51, 2096–2102. [Google Scholar] [CrossRef]
- Posner, T.B.; Hall, C.D. 1H and 13C nuclear magnetic resonance spectra of benzylidenemalononitriles: A method for the determination of σ+ substituent constants. J. Chem. Soc. Perkin Trans. II 1976, 6, 729–732. [Google Scholar] [CrossRef]
- Pádua, A.; Costa, M.F.; Canongia, J.N.A. Molecular solutes in ionic liquids: A structural perspective. Acc. Chem. Res. 2007, 40, 1087–1096. [Google Scholar] [CrossRef]
- Annapureddy, H.V.R.; HU, Z.H.; Xia, J.C.; Margulis, C.J. How does water affect the dynamics of the room-temperature ionic liquid 1-hexyl-3-methylimidazolium hexafluorophosphate and the fluorescence spectroscopy of coumarin-153 when dissolved in it? J. Phys. Chem. B 2008, 112, 1770–1776. [Google Scholar] [CrossRef]
- Klähn, M.; Stüber, C.; Seduraman, A.; Wu, P. What determines the miscibility of ionic liquids with water? Identification of the underlying factors to enable a straightforward prediction. J. Phys. Chem. B 2010, 114, 2856–2868. [Google Scholar] [CrossRef]
- Aggarwal, A.; Lancaster, N.L.; Sethi, A.R.; Welton, T. The role of hydrogen bonding in controlling the selectivity of Diels-Alder reactions in room-temperature ionic liquids. Green Chem. 2002, 4, 517–520. [Google Scholar] [CrossRef]
- Roy, S.R.; Chakraborti, A.K. Supramolecular assemblies in ionic liquid catalysis for Aza-Michael reaction. Org. Lett. 2010, 12, 3866–3869. [Google Scholar]
- Rong, L.; Li, X.; Wang, H.; Shi, D.; Tu, S.; Zhuang, Q. Efficient green procedure for the Knoevenagel condensation under solvent-free conditions. Syn. Commun. 2006, 36, 2407–2412. [Google Scholar] [CrossRef]
- El-Deen, I.M.; Ibrahim, H.K. Synthesis and electron impact of mass spectra of 3-substituted chromeno[3,2-c]chromene-6,7-diones. Chem. Papers 2004, 58, 200–204. [Google Scholar]
- Corbu, A.; Pérez, M.; Aquino, M.; Retailleau, P.; Arseniyadis, S. Total synthesis and structural confirmation of ent-galbanic acid (I) and marneral (II). Org. Lett. 2008, 10, 2853–2856. [Google Scholar] [CrossRef]
- Karade, N.N.; Gampawar, S.V.; Shinde, S.V.; Jadhav, W.N. L-proline catalyzed solvent-free knoevenagel condensation for the synthesis of 3-substituted coumarins. Chinese J. Chem. 2007, 25, 1686–1689. [Google Scholar]
- Bowman, M.D.; Schmink, J.R.; McGowan, C.M.; Kormos, C.M.; Leadbeater, N.E. Scale-up of microwave-promoted reactions to the multigram level using a sealed-vessel microwave apparatus. Org. Process. Res. Dev. 2008, 12, 1078–1088. [Google Scholar] [CrossRef]
- Moussaoui, Y.; Ben Salem, R.C.R. Catalyzed Knoevenagel reactions on inorganic solid supports: Application to the synthesis of coumarin compounds. Chimie 2007, 10, 1162–1169. [Google Scholar] [CrossRef]
- Alvim, J., Jr.; Dias, R.L.A.; Castilho, M.S.; Oliva, G.; Correa, A.G.J. Preparation and evaluation of a coumarin library towards the inhibitory activity of the enzyme gGAPDH from Trypanosoma cruzi. Braz. Chem. Soc. 2005, 16, 763–773. [Google Scholar] [CrossRef]
- Surya Prakash Rao, H.; Sivakumar, S. Condensation of α-aroylketene dithioacetals and 2-hydroxyarylaldehydes results in facile synthesis of a combinatorial library of 3-aroylcoumarins. J. Org. Chem. 2006, 71, 8715–8723. [Google Scholar] [CrossRef]
- Chimenti, F.; Secci, D.; Bolasco, A.; Chimenti, P.; Granese, A.; Befani, O.; Turini, P.; Alcaro, S.; Ortuso, F. Inhibition of monoamine oxidases by coumarin-3-acyl derivatives: Biological activity and computational study. Bioorg. Medl. Chem. Lett. 2004, 14, 3697–3703. [Google Scholar] [CrossRef]
- Yavari, I.; Djahaniani, H.; Nasiri, F. Synthesis of coumarins and 4H-chromenes through the reaction of tert-butyl isocyanide and dialkyl acetylenedicarboxylates in presence of 2-hydroxybenzaldehydes. Synthesis 2004, 5, 679–682. [Google Scholar]
- Murata, C.; Masuda, T.; Kamochi, Y.; Todoroki, K.; Yoshida, H.; Nohta, H.; Yamaguchi, M.; Takadate, A. Improvement of fluorescence characteristics of coumarins: Syntheses and fluorescence properties of 6-methoxycoumarin and benzocoumarin derivatives as novel fluorophores emitting in the longer wavelength region and their application to analytical reagents. Chem. Pharm. Bull. 2005, 53, 750–758. [Google Scholar] [CrossRef]
- Valizadeh, H.; Shockravi, A.; Gholipur, H. Microwave assisted synthesis of coumarins via potassium carbonate catalyzed Knoevenagel condensation in 1-n-butyl-3-methylimidazolium bromide ionic liquid. J. Het. Chem. 2007, 44, 867–870. [Google Scholar] [CrossRef]
- Merchant, J.R.; Shah, P.J. Synthesis of 3-coumaryl phenyl sulfones or sulfoxides. J. Het. Chem. 1981, 18, 441–442. [Google Scholar]
- El-Shafei, A.; Fadda, A.A.; Abdel-Gawad, I.I.; Youssif, E.H.E. Stereospecificity of Diels-Alder reactions validated using Ab initio calculations: Synthesis of novel coumarin and phenanthridine derivatives. Synth. Commun. 2009, 39, 2954–2972. [Google Scholar]
- García-Lorenzo, A.; Tojo, E.; Tojo, J.; Teijeira, M.; Rodríguez-Berrocal, F.J.; Pérez González, M.; Martínez-Zorzano, V.S. Cytotoxicity of selected imidazolium-derived ionic liquids in the human Caco-2 cell line. Sub-structural toxicological interpretation through a QSAR study. Green Chem. 2008, 10, 508–516. [Google Scholar] [CrossRef]
- Sample Availability: Samples of all the compounds are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Verdía, P.; Santamarta, F.; Tojo, E. Knoevenagel Reaction in [MMIm][MSO4]: Synthesis of Coumarins. Molecules 2011, 16, 4379-4388. https://doi.org/10.3390/molecules16064379
Verdía P, Santamarta F, Tojo E. Knoevenagel Reaction in [MMIm][MSO4]: Synthesis of Coumarins. Molecules. 2011; 16(6):4379-4388. https://doi.org/10.3390/molecules16064379
Chicago/Turabian StyleVerdía, Pedro, Francisco Santamarta, and Emilia Tojo. 2011. "Knoevenagel Reaction in [MMIm][MSO4]: Synthesis of Coumarins" Molecules 16, no. 6: 4379-4388. https://doi.org/10.3390/molecules16064379