Catalytic Synthesis of α-Oxoketene S,S-Acetals in a Wet Ionic Liquid [Bmim]Cl/H2O Homogeneous System
Abstract
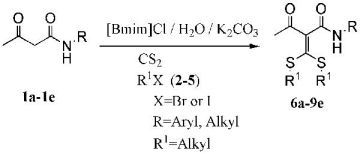
:1. Introduction
2. Results and Discussion
2.1. Choice of Ionic Liquids
2.2. The Ratio of [Bmim]Cl to Substrate
2.3. Recycling of the [Bmim]Cl/water
2.4. The Synthesis of Different α-oxoketene S,S-acetals
3. Experimental
3.1. Preparation of Materials
3.2. General Procedure for the Preparation of α-oxoketene S,S-acetals 6a-7a, 7b-9b and 6c-7c
3.3. General Procedure for the Preparation of α-oxoketene S,S-acetals 7d-9d and 7e-9e
4. Conclusions
Acknowledgments
References
- Kumar, S.; Peruncheralathan, S.; Ila, H.; Junjappa, H. A Novel Anionic Domino Process for the Synthesis of o-Cyanoaryl-Methylthio/Alkyl/Aryl/Heteroaryl Acetylenes. Org. Lett. 2008, 10, 965–968. [Google Scholar] [CrossRef] [PubMed]
- Sasikala, K.A.; Kalesh, K.A.; Anabha, E.R.; Pillai, P.M.; Asokan, C.V.; Devaky, K.S. Synthesis of 2,3,5-trisubstituted furans from [alpha]-formylaroylketene dithioacetals. Tetrahedron Lett. 2011, 52, 1667–1669. [Google Scholar] [CrossRef]
- Li, Y.; Liang, F.; Bi, X.; Liu, Q. Intramolecular Thia-anti-Michael Addition of a Sulfur Anion to Enones: A Regiospecific Approach to Multisubstituted Thiophene Derivatives. J. Org. Chem. 2006, 71, 8006–8010. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.; Dong, D.; Liu, Q.; Pan, W.; Zhao, L.; Li, B. [5 + 1] Annulation: A Synthetic Strategy for Highly Substituted Phenols and Cyclohexenones. J. Am. Chem. Soc. 2005, 127, 4578–4579. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Fu, Z.; Feng, H.; Dong, Y.; Liu, J.; Liu, Q. Tandem [4 + 1 + 1] annulation and metal-free aerobic oxidative aromatization: straightforward synthesis of highly substituted phenols from one aldehyde and two ketones. Chem. Commun. 2010, 46, 9061–9063. [Google Scholar] [CrossRef] [PubMed]
- Kelber, C.; Dtsch, B. Über die Einwirkung von Schwefelkohlenstoff und Ätzkali auf Acetophenon. Chem. Ges. 1910, 43, 1252–1259. [Google Scholar] [CrossRef]
- Choi, E.B.; Youn, I.K.; Pak, C.S. Preparation of Protected β- Keto Esters via Selective Reduction of Acyl(alkoxycarbonyl)ketene Dithioacetals. Synthesis 1988, 792–794. [Google Scholar] [CrossRef]
- Ouyang, Y.; Dong, D.; Yu, H.; Liang, Y.; Liu, Q. A Clean, Facile and Practical Synthesis of α-Oxoketene S,S-Acetals in Water. Adv. Synth. Catal. 2006, 348, 206–210. [Google Scholar] [CrossRef]
- Muthusamy, S.; Gnanaprakasam, B. Imidazolium salts as phase transfer catalysts for the dialkylation and cycloalkylation of active methylene compounds. Tetrahedron Lett. 2005, 46, 635–638. [Google Scholar] [CrossRef]
- Welton, T. Room-Temperature Ionic Liquids. Solvents for Synthesis and Catalysis. Chem. Rev. 1999, 99, 2071–2084. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Zhou, Q.; He, X.; Wei, S.; Wang, L.; van Kasteren, J.M.N.; Wang, Y.Z. A highly efficient approach for dehydrochlorinating polyvinyl chloride: Catalysis by 1-butyl-3-methylimidazolium chloride. Green Chem. 2010, 12, 1062–1065. [Google Scholar] [CrossRef]
- Bai, S.; Song, B.; Bhadury, P.S.; Yang, S.; Hu, D.; Xue, W. [BMIM]Cl Catalyzed One-Pot Synthesis of α-Aminophosphonate Derivatives Containing a 4-Phenoxyquinazoline Moiety under Microwave Irradiation. Chin. J. Chem. 2011, 29, 109–117. [Google Scholar] [CrossRef]
- Sun, H.; Li, X.; Sundermeyer, J. Aerobic oxidation of phenol to quinone with copper chloride as catalyst in ionic liquid. J. Mol. Catal. A Chem. 2005, 240, 119–122. [Google Scholar] [CrossRef]
- Li, H.; Zhu, W.; Wang, Y.; Zhang, J.; Lu, J.; Yan, Y. Deep oxidative desulfurization of fuels in redox ionic liquids based on iron chloride. Green Chem. 2009, 11, 810–815. [Google Scholar] [CrossRef]
- Yuan, X.; Chen, M.; Dai, Q.; Cheng, X. Friedel-Crafts acylation of anthracene with oxalyl chloride catalyzed by ionic liquid of [bmim]Cl/AlCl3. Chem. Eng. J. 2009, 146, 266–269. [Google Scholar]
- Liu, F.; Li, Z.; Yu, S.; Cui, X.; Ge, X. Environmentally benign methanolysis of polycarbonate to recover bisphenol A and dimethyl carbonate in ionic liquids. J. Hazard. Mater. 2010, 174, 872–875. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Wu, C.; Christopher, B.W.; Quan, J.; Zhu, L. Ionic Liquids—Promoted S-Methylation of Thiols Utilizing Dimethyl Carbonate. Phosphorus, Sulfur Silicon Relat. Elem. 2011, 186, 31–37. [Google Scholar] [CrossRef]
- Tilve, R.D.; Alexander, M.V.; Khandekar, A.C.; Samant, S.D.; Kanetkar, V.R. Synthesis of 2,3-unsaturated glycopyranosides by Ferrier rearrangement in FeCl3 based ionic liquid. J. Mol. Catal. A: Chem. 2004, 223, 237–240. [Google Scholar] [CrossRef]
- Qi, X.; Watanabe, M.; Aida, T.M.; Smith, R.L. Efficient Catalytic Conversion of Fructose into 5-Hydroxymethylfurfural in Ionic Liquids at Room Temperature. ChemSusChem 2009, 2, 944–946. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wang, S.J.; Yu, C.S.; Ma, Y.C.; Li, K.L.; Lin, L.W. Direct conversion of methane to methanol over nano-[Au/SiO2] in [Bmim]Cl ionic liquid. Appl. Catal. A Gen. 2011, 398, 150–154. [Google Scholar] [CrossRef]
- Qi, X.; Watanabe, M.; Aida, T.M.; Smith, J.R.L. Efficient process for conversion of fructose to 5-hydroxymethylfurfural with ionic liquids. Green Chem. 2009, 11, 1327–1331. [Google Scholar] [CrossRef]
- Brown, R.A.; Pollet, P.; McKoon, E.; Eckert, C.A.; Liotta, C.L.; Jessop, P.G. Asymmetric Hydrogenation and Catalyst Recycling Using Ionic Liquid and Supercritical Carbon Dioxide. J. Am. Chem. Soc. 2001, 123, 1254–1255. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Liu, Y.; Shi, S.; Wang, Y. A simple, efficient and green procedure for Knoevenagel condensation catalyzed by [C4dabco][BF4] ionic liquid in water. Green Chem. 2010, 12, 514–517. [Google Scholar] [CrossRef]
- De Nino, A.; Bortolini, O.; Maiuolo, L.; Garofalo, A.; Russo, B.; Sindona, G. A sustainable procedure for highly enantioselective organocatalyzed Diels-Alder cycloadditions in homogeneous ionic liquid/water phase. Tetrahedron Lett. 2011, 52, 1415–1417. [Google Scholar] [CrossRef]
- Odinets, I.L.; Matveeva, E.V. Ionic Liquids and Water as “Green” Solvents in Organophosphorus Synthesis. Curr. Org. Chem. 2010, 14, 1171–1184. [Google Scholar] [CrossRef]
- Fang, D.; Zhang, D.; Liu, Z. One-pot three-component Biginelli-type reaction catalyzed by ionic liquids in aqueous media. Monatsh. Chem. 2010, 141, 419–423. [Google Scholar] [CrossRef]
- Gutowski, K.E.; Broker, G.A.; Willauer, H.D.; Huddleston, J.G.; Swatloski, R.P.; Holbrey, J.D.; Rogers, R.D. Controlling the Aqueous Miscibility of Ionic Liquids: Aqueous Biphasic Systems of Water-Miscible Ionic Liquids and Water-Structuring Salts for Recycle, Metathesis, and Separations. J. Am. Chem. Soc. 2003, 125, 6632–6633. [Google Scholar] [CrossRef] [PubMed]
- Mele, A.; Tran, C.D.; De Paoli Lacerda, S.H. The Structure of a Room-Temperature Ionic Liquid with and without Trace Amounts of Water: The Role of C H O and C H F Interactions in 1-n-Butyl-3-Methylimidazolium Tetrafluoroborate. Angew. Chem. Int. Ed. 2003, 42, 4364–4366. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, A.W. Ueber zwei neue Reihen flüchtiger organischer Basen. Justus Liebigs Annalen der Chemie 1850, 73, 91–92. [Google Scholar] [CrossRef]
- Hofmann, A.W. Beiträge zur Kenntniss der flüchtigen organischen Basen. Justus Liebigs Annalen der Chemie 1851, 79, 11–39. [Google Scholar] [CrossRef]
- Raleigh, E.W.; Wilmington, D. Preparation of 1-(carbamoyl)-N-(carbamoyloxy)thioformimidates from acetoacetamides. U.S. Patent 3,557,089, 19 January 1971. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |


| Entry | Reaction Medium | Amount a (mmol)/(mL) | K2CO3 (mmol) | 1a (mmol) | Time b (h) | Yield c (%) |
|---|---|---|---|---|---|---|
| 1 | H2O | 15 (mL) | 12.5 | 5 | 20 | 0 |
| 2 | [EPy]BF4 | 50 (mmol) | 12.5 | 5 | 20 | trace |
| 3 | [Bmim]Cl | 50 (mmol) | 12.5 | 5 | 20 | trace |
| 4 | [EPy]BF4/H2O | 50/15 | 12.5 | 5 | 15 | 71 |
| 5 | [Bmim]Cl/H2O | 50/15 | 12.5 | 5 | 5 | 73 |
| 6 | [Bmim]Cl/H2O | 40/15 | 12.5 | 5 | 5 | 74 |
| 7 | [Bmim]Cl/H2O | 30/15 | 12.5 | 5 | 5 | 73 |
| 8 | [Bmim]Cl/H2O | 20/15 | 12.5 | 5 | 5 | 74 |
| 9 | [Bmim]Cl/H2O | 10/15 | 12.5 | 5 | 8 | 65 |
| 10 | [Bmim]Cl/H2O | 5/15 | 12.5 | 5 | 11 | 61 |
| 11 | [Bmim]Cl/H2O | 0.1/15 | 12.5 | 5 | 20 | 43 |
| Entry | Reuse times | Reaction medium | 1a (mmol) | CS2 (mmol) | BrCH2CH2Br (mmol) | Time a (h) | Yield b (%) |
|---|---|---|---|---|---|---|---|
| 1 | 2 | [Bmim]Cl/H2O c | 5 | 6 | 5 | 5 | 70 |
| 2 | 3 | [Bmim]Cl/H2O d | 5 | 6 | 5 | 6 | 65 |
| 3 | 4 | [Bmim]Cl/H2O e | 5 | 6 | 5 | 6 | 65 |
| 4 | 5 | [Bmim]Cl/H2O f | 5 | 6 | 5 | 7 | 66 |

| Entry | Substrate | Substrate | Time a (h) | Product | Yield b (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| 1 R | 2–5 | X R1 | R1 | ||||||
| 1 | 1a | C6H5 | 2 | Br | -(CH2)3- | 5 | 6a | 73 | |
| 2 | 1a | C6H5 | 3 | Br | -(CH2)2- | 5 | 7a | 74 | |
| 3 | 1b | CH(CH2)5 | 3 | Br | -(CH2)2- | 6 | 7b | 60 | |
| 4 | 1b | CH(CH2)5 | 4 | Br | -C2H5 | -C2H5 | 6 | 8b | 55 |
| 5 | 1b | CH(CH2)5 | 5 | I | -CH3 | -CH3 | 6 | 9b | 53 |
| 6 | 1c | o-Methylphenyl | 2 | Br | -(CH2)3- | 6 | 6c | 70 | |
| 7 | 1c | o-Methylphenyl | 3 | Br | -(CH2)2- | 6 | 7c | 72 | |
| 8 | 1d | -CH3 | 3 | Br | -(CH2)2- | 6 | 7d | 65 | |
| 9 | 1d | -CH3 | 4 | Br | -C2H5 | -C2H5 | 6 | 8d | 61 |
| 10 | 1d | -CH3 | 5 | I | -CH3 | -CH3 | 6 | 9d | 60 |
| 11 | 1e | -(CH2)3CH3 | 3 | Br | -(CH2)2- | 6 | 7e | 66 | |
| 12 | 1e | -(CH2)3CH3 | 4 | Br | -C2H5 | -C2H5 | 6 | 8e | 55 |
| 13 | 1e | -(CH2)3CH3 | 5 | I | -CH3 | -CH3 | 6 | 9e | 58 |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yu, P.; Zu, Y.; Fu, Y.; Efferth, T. Catalytic Synthesis of α-Oxoketene S,S-Acetals in a Wet Ionic Liquid [Bmim]Cl/H2O Homogeneous System. Molecules 2011, 16, 4500-4510. https://doi.org/10.3390/molecules16064500
Yu P, Zu Y, Fu Y, Efferth T. Catalytic Synthesis of α-Oxoketene S,S-Acetals in a Wet Ionic Liquid [Bmim]Cl/H2O Homogeneous System. Molecules. 2011; 16(6):4500-4510. https://doi.org/10.3390/molecules16064500
Chicago/Turabian StyleYu, Ping, Yuangang Zu, Yujie Fu, and Thomas Efferth. 2011. "Catalytic Synthesis of α-Oxoketene S,S-Acetals in a Wet Ionic Liquid [Bmim]Cl/H2O Homogeneous System" Molecules 16, no. 6: 4500-4510. https://doi.org/10.3390/molecules16064500