Maleimido-Functionalized NOTA Derivatives as Bifunctional Chelators for Site-Specific Radiolabeling
Abstract
:1. Introduction
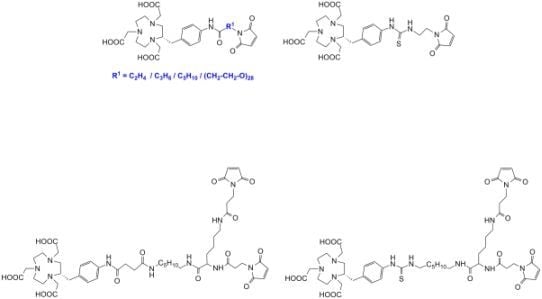
2. Results and Discussion





3. Experimental
3.1. Materials
3.2. Chromatographic Purifications and Analyses
3.3. General Procedure for the Syntheses of Thiourea Bound NOTA-Maleimides
3.4. General Procedure for the Syntheses of Amide Bound NOTA-Maleimides
4. Conclusions
References and Notes
- Wieghardt, K.; Bossek, U.; Ghaudhuri, P.; Herrmann, W.; Menke, B.C.; Weiss, J. 1,4,7-Triazacyclononane-N,N’,N’’-triacetate (TCTA), a new hexadentate ligand for divalent and trivalent metal ions. Crystal structures of [CrIII(TCTA)], [FeIII(TCTA)], and Na[CuII(TCTA)]*2NaBr*8H2O. Inorg. Chem. 1982, 21, 4308–4314. [Google Scholar] [CrossRef]
- Cacharis, W.P.; Nickle, S.K.; Sherry, A.D. Thermodynamic study of lanthanide complexes of 1,4,7-triazacyclononane-N,N’,N’’-triacetic acid and 1,4,7,10-tetraazacyclododecane-N,N’,N’’,N’’’-tetraacetic acid. Inorg. Chem. 1987, 26, 958–960. [Google Scholar] [CrossRef]
- Boeyens, J.C.A.; van der Merwe, M.J. The nonexistent crystals of macrocyclic nickel(III). Structure of the cobalt(III) complex of 1,4,7-triazacyclononane-N,N’,N’’-triacetate. Inorg. Chem. 1997, 36, 3779–3780. [Google Scholar] [CrossRef]
- Clarke, E.T.; Martell, A.E. Stabilities of the Fe(III), Ga(III) and In(III) chelates of N,N’,N’’-triazacyclononanetriacetic acid. Inorg. Chim. Acta 1991, 181, 273–280. [Google Scholar] [CrossRef]
- Craig, A.S.; Parker, D.; Adams, H.; Bailey, N.A. Stability, 71Ga NMR, and crystal structure of a neutral gallium(III) complex of 1,4,7-triazacyclononanetriacetate: A potential radio-pharmaceutical? J. Chem. Soc. Chem. Commun. 1989, 1793–1794. [Google Scholar]
- McMurry, T.J.; Brechbiel, M.; Wu, C.; Gansow, O.A. Synthesis of 2-(p-thiocyanatobenzyl)-1,4,7-triazacyclononane-1,4,7-triacetic acid: Application of the 4-methoxy-2,3,6-trimethylbenzene-sulfonamide protecting group in the synthesis of macrocyclic polyamines. Bioconj. Chem. 1993, 4, 236–245. [Google Scholar] [CrossRef]
- Andre, J.P.; Maecke, H.R.; Zehnder, M.; Macko, L.; Akyel, K.G. 1,4,7-Triazacyclononane-1-succinic acid-4,7-diacetic acid (NODASA): A new bifunctional chelator for radio gallium-labelling of biomolecules. Chem. Commun. 1998, 1301–1302. [Google Scholar]
- de Sa, A.; Matias, A.A.; Prata, M.I.M.; Geraldes, C.F.G.C.; Ferreira, P.M.T.; Andre, J.P. Gallium labeled NOTA-based conjugates for peptide receptor-mediated medical imaging. Bioorg. Med. Chem. Lett. 2010, 24, 7345–7348. [Google Scholar]
- Wagner, S.J.; Welch, M.J. Gallium-68 labeling of albumin and albumin microspheres. J. Nucl. Med. 1979, 20, 428–433. [Google Scholar]
- Endo, N.; Kato, Y.; Takeda, Y.; Saito, M.; Umemoto, K.; Kishida, K.; Hara, T. In vitro cytotoxicity of a human serum albumin-mediated conjugate of methotrexate with anti-MM46 monoclonal antibody. Cancer Res. 1987, 47, 1076–1080. [Google Scholar]
- Ahlgren, S.; Andersson, K.; Tolmachev, V. Kit formulation for 99mTc-labeling of recombinant anti-HER2 affibody molecules with a C-terminally engineered cysteine. Nucl. Med. Biol. 2010, 37, 539–546. [Google Scholar] [CrossRef]
- Ahlgren, S.; Orlova, A.; Rosik, D.; Sandström, M.; Sjöberg, A.; Baastrup, B.; Widmark, O.; Fant, G.; Feldwisch, J.; Tolmachev, V. Evaluation of maleimide derivative of DOTA for site-specific labeling of recombinant affibody molecules. Bioconj. Chem. 2008, 19, 235–243. [Google Scholar] [CrossRef]
- Wåhlberg, H.; Ahlgren, S.; Widström, C.; Orlova, A. Evaluation of the radiocobalt-labeled [MMA-DOTA-Cys62]-THER2:2395-Cys affibody, olecule for targeting of HER2-expressing tumors. Mol. Imaging Biol. 2010, 12, 54–62. [Google Scholar] [CrossRef]
- McBride, W.J.; D’Souza, C.A.; Sharkey, R.M.; Karacay, H.; Rossi, E.A.; Chang, C.-H.; Goldenberg, D.M. Improved 18F labeling of peptides with a fluoride-aluminum-chelate complex. Bioconj. Chem. 2010, 21, 1331–1340. [Google Scholar] [CrossRef]
- Cox, J.P.L.; Craig, A.S.; Helps, I.M.; Jankowski, K.J.; Parker, D.; Eaton, M.A.W.; Millican, A.T.; Millar, K.; Beeley, N.R.A.; Boyce, B.A. Synthesis of C- and N-functionalised derivatives of 1,4,7-triazacyclononane-1,4,7-triyltriacetic acid (NOTA), 1,4,7,10-tetra-azacyclododecane-1,4,7,10-tetrayltetra-acetic acid DOTA), and diethylenenetriaminepenta-acetic acid (DTPA): Bifunctional complexing agents for the derivatisation of antibodies. J. Chem. Soc. Perkin Trans. 1990, 1, 2567–2576. [Google Scholar]
- Eder, M.; Krivoshein, A.V.; Backer, M.; Backer, J.M.; Haberkorn, U.; Eisenhut, M. ScVEGF-PEGHBED-CC and scVEGF-PEG-NOTA conjugates: comparision of easy-to-label recombinant proteins for [68Ga]PEG imaging of VEGF receptors in angiogenic vasculature. Nucl. Med. Biol. 2010, 37, 405–412. [Google Scholar] [CrossRef]
- Tolmachev, V.; Altai, M.; Sandström, A.; Perols, A.; Karlström, A.K.; Boschetti, F.; Orlova, A. Evaluation of a maleimido derivative of NOTA for site specific labeling of affibody molecules. Bioconj. Chem. 2011, 22, 894–902. [Google Scholar] [CrossRef]
- Li, Z.-B.; Chen, K.; Chen, X. 68Ga-labeled multimeric RGD peptides for microPET imaging of integrin αVß3 expression. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1100–1108. [Google Scholar] [CrossRef]
- Kubas, H.; Schäfer, M.; Bauder-Wüst, U.; Eder, M.; Oltmanns, D.; Haberkorn, U.; Mier, W.; Eisenhut, M. Multivalent cyclic RGD ligands: Influence of linker length on receptor bindung. Nucl. Med. Biol. 2010, 37, 885–891. [Google Scholar] [CrossRef]
- Vada, O.; Hartley, O.; Rose, K. Characterization of new multimeric erythropoietin receptors agonists. Biopolymers 2008, 90, 496–502. [Google Scholar] [CrossRef]
- Monfardini, C.; Veronese, F.M. Stabilization of substances in circulation. Bioconj. Chem. 1998, 9, 418–450. [Google Scholar] [CrossRef]
- Schlesinger, J.; Bergmann, R.; Klussmann, S.; Wüst, F. Synthesis and radiopharmacological characterization of Y-86 and Ga-68-labelled L-RNA oligonucleotides as molecular probes for positron emission tomography (PET). Lett. Drug Des. 2006, 3, 330–335. [Google Scholar] [CrossRef]
- Schlesinger, J.; Koetzle, I.; Bergamann, R.; Tamburini, S.; Bolzati, C.; Tisato, F.; Noll, B.; Klussmann, S.; Vonhoff, S.; Wüst, F.; Pietzsch, H.J.; Steinbach, J. An Y-labeled mirror-image oligonucleotide: Influence of Y-DOTA Isomers on the biodistribution in rats. Bioconj. Chem. 2008, 19, 928–939. [Google Scholar] [CrossRef]
- Boswell, C.A.; Tesar, D.B.; Mukhyala, K.; Theil, F.-P.; Fielder, P.J.; Khawli, L.A. Effect of charge on antibody tissue distribution and pharmacokinetics. Bioconj. Chem. 2010, 21, 2153–2163. [Google Scholar] [CrossRef]
- Jevsêvar, S.; Kunstelj, M.; Porekar, V.G. PEGylation of therapeutic proteins. Biotechnol. J. 2010, 5, 113–128. [Google Scholar]
- Knop, K.; Hoogenboom, R.; Fischer, D.; Schubert, U.S. Poly(ethylene glycol) in drug delivery: Pros and cons as well as potential alternatives. Angew. Chem. Int. Ed. 2010, 49, 6288–6308. [Google Scholar] [CrossRef]
- Samples Availability: Samples Not Available.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Förster, C.; Schubert, M.; Pietzsch, H.-J.; Steinbach, J. Maleimido-Functionalized NOTA Derivatives as Bifunctional Chelators for Site-Specific Radiolabeling. Molecules 2011, 16, 5228-5240. https://doi.org/10.3390/molecules16065228
Förster C, Schubert M, Pietzsch H-J, Steinbach J. Maleimido-Functionalized NOTA Derivatives as Bifunctional Chelators for Site-Specific Radiolabeling. Molecules. 2011; 16(6):5228-5240. https://doi.org/10.3390/molecules16065228
Chicago/Turabian StyleFörster, Christian, Maik Schubert, Hans-Jürgen Pietzsch, and Jörg Steinbach. 2011. "Maleimido-Functionalized NOTA Derivatives as Bifunctional Chelators for Site-Specific Radiolabeling" Molecules 16, no. 6: 5228-5240. https://doi.org/10.3390/molecules16065228