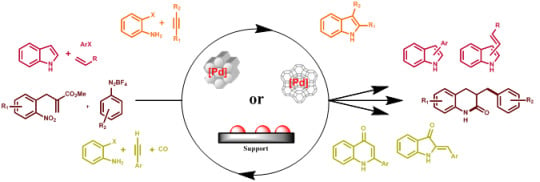
Recent Advances in the Synthesis of N-Containing Heteroaromatics via Heterogeneously Transition Metal Catalysed Cross-Coupling Reactions
Abstract
:1. Introduction
2. Selective Transformation of Pre-Existing N-Containing Heterocycles

2.1. Selective Alkenylation of Indoles
| Entry | Catalyst | Conv. (20 h) b | Sel. C2/C3 c | Ai d |
|---|---|---|---|---|
| 1 | [Pd(OAc)2/Cu(OAc)2] | 52% | 0/100 | 0.9 × 10−3 |
| 2 | [PdCl(hp)3Cu]2 | 65% | 0/100 | 0.4 × 10−3 |
| 3 | [Pd/Cu]/NaY | 60% | 0/100 | 0.4 × 10−3 |
| Entry | R | Conv. (1 h) b | C3-selectivity c |
|---|---|---|---|
| 1 | H | 51 % | 100% |
| 2 | Me | 88 % | 100% |
| 3 | Ph | 56 % | 100% |
| 4 | CO2Me | 28 % | 76% d |
2.2. Selective Arylation of Indoles
2.2.1. N1-arylation



2.2.2. C2 and C3-arylation


3. Selective Construction of N-Containing Heterocycles
3.1. Selective Syntheses of Indoles
3.1.1. Through one-step procedures





3.1.2. Through one-pot multi-step procedures







3.2. Selective Syntheses of 2-Quinolones


3.3. Selective Syntheses of 4-Quinolones


3.4. Selective Syntheses of Indoxyls

3.5. Selective Syntheses of Dihydroisoquinolin-3-ones

4. Conclusions
Acknowledgments
Conflict of Interest
References and Notes
- Sundberg, R.J. Indoles; Academic Press: London, UK, 1996. [Google Scholar]
- Joule, J.A. Product Class 13: Indole and Its Derivatives; Thieme: Stuttgart, Germany, 2001; Volume 10, pp. 361–652. [Google Scholar]
- Grohe, K. Antibiotics-The new generation. Chem. Brit. 1992, 28, 34–36. [Google Scholar]
- Wentland, M.P.; Cornett, J.B. Quinolone antibacterial agents. Ann. Rpt. Med. Chem. 1985, 20, 145–154. [Google Scholar] [CrossRef]
- Aimi, A.; Nishimura, M.; Miwa, A.; Hoshino, H.; Sakai, S.; Higiniwa, J. Pumiloside and deoxypumiloside; plausible intermediates of camptothecin biosynthesis. Tetrahedron Lett. 1989, 30, 4991–4994. [Google Scholar] [CrossRef]
- Sundberg, R.J. Pyrroles and their Benzo Derivatives: Synthesis and Applications; Pergamon: Oxford, UK, 1984; Volume 4, pp. 313–376. [Google Scholar]
- Tois, J.; Franzén, R.; Koskinen, A. Synthetic approaches towards indoles on solid phase recent advances and future directions. Tetrahedron 2003, 59, 5395–5405. [Google Scholar] [CrossRef]
- Joucla, L.; Djakovitch, L. Transition metal-catalysed, direct and site-selective N1-, C2- or C3-arylation of the indole nucleus: 20 years of improvement. Adv. Synth. Catal. 2009, 351, 673–714. [Google Scholar] [CrossRef]
- Robinson, B. The Fischer Indole Synthesis; John Wiley & Sons: Chichester, UK, 1982. [Google Scholar]
- Leonard, N.J.; Herbrandson, F.-F.; van Heyningen, E.M. Synthesis of 4-hydroxyquinolines. VI. Synthesis of 3-normal-heptyl-7-methoxy-2-methyl-4-quinolinol. J. Am. Chem. Soc. 1946, 68, 1279–1281. [Google Scholar] [CrossRef]
- Reitsema, R.H. The chemistry of 4-hydroxyquinolines. Chem. Rev. 1948, 43, 43–68. [Google Scholar] [CrossRef]
- Chen, B.; Huang, X.; Wang, J. A versatile synthesis of 2-alkyl and 2-aryl 4-quinolones. Synthesis 1987, 1987, 482–484. [Google Scholar] [CrossRef]
- Kasahara, A.; Izumi, T.; Watabe, H.; Takahashi, S. A new synthesis of 2-aryl-4-quinolones. Chem. Ind. 1981, 121. [Google Scholar]
- Hughes, D.L. Progress in the Fischer indole reaction-A review. Org. Prep. Proced. Int. 1993, 25, 609–632. [Google Scholar]
- Clark, R.D.; Repke, D.B. The Leimgruber-Batcho indole synthesis. Heterocycles 1984, 22, 195–221. [Google Scholar] [CrossRef]
- Wright, S.W.; McClure, L.D.; Hageman, D.L. A convenient modification of the Gassman oxindole synthesis. Tetrahedron Lett. 1996, 37, 4631–4634. [Google Scholar] [CrossRef]
- Johnson, P.D.; Aristoff, P.A. General procedure for the synthesis of o-aminophenylacetates by a modification of the Gassman reaction. J. Org. Chem. 1990, 55, 1374–1375. [Google Scholar] [CrossRef]
- Wacker, D.A.; Kasireddy, P. Efficient solid-phase synthesis of 2,3-substituted indoles. Tetrahedron Lett. 2002, 43, 5189–5191. [Google Scholar] [CrossRef]
- Saheb, S.E.; Haddadin, M.J. Stable, biologically active indoxyl. J. Med. Chem. 1971, 14, 445–446. [Google Scholar] [CrossRef]
- Ardakani, M.A.; Smalley, R.K. Base-induced intramolecular cyclization of o-azidophenyl sec-alkyl ketones. A new synthesis of 2,2-dialkylindoxyls. Tetrahedron Lett. 1979, 20, 4769–4772. [Google Scholar] [CrossRef]
- Flowers, W.T.; Holt, G.; Poulos, C.P.; Poulos, K. Novel aspects in the synthesis and acid-promoted cyclization of some 2-diazo-2'-(p-tolylsulfonylamino)acetophenones: Electrophilic displacement of an arylsulfonyl group from a sulfonamide. J. Chem. Soc. Perkin Trans. 1 1976, 1757–1762. [Google Scholar]
- Zeni, G.; Larock, R.C. Synthesis of heterocycles via palladium-catalyzed oxidative addition. Chem. Rev. 2006, 106, 4644–4680. [Google Scholar] [CrossRef]
- Cacchi, S.; Fabrizi, G.; Lamba, D.; Marinelli, F.; Parisi, L.M. 2-Substituted 3-aryl- and 3-heteroarylindoles by the palladium-catalyzed reaction of o-trifluoroacetanilides with aryl bromides and triflates. Synthesis 2003, 728–734. [Google Scholar]
- Okuro, K.; Alper, H. Palladium-catalyzed carbonylation of o-iodophenols with allenes. J. Org. Chem. 1997, 62, 1566–1567. [Google Scholar] [CrossRef]
- Grigg, R.; Liu, A.; Shaw, D.; Suganthan, S.; Woodall, D.E.; Yoganathan, G. Synthesis of quinol-4-ones and chroman-4-ones via a palladium-catalyzed cascade carbonylation-allene insertion. Tetrahedron Lett. 2000, 41, 7125–7128. [Google Scholar]
- Torii, S.; Okumoto, H.; Xu, L.H. Palladium-catalyzed carbonylation to form 2-substituted 1,4-dihydro-4-oxo-quinoline. Tetrahedron Lett. 1991, 32, 237–240. [Google Scholar] [CrossRef]
- Torii, S.; Okumoto, H.; Xu, L.H.; Sadakane, M.; Shostakovsky, M.V.; Ponomaryov, A.B.; Kalinin, V.N. Syntheses of chromones and quinolones via pd-catalyzed carbonylation of o-iodophenols and anilines in the presence of acetylenes. Tetrahedron 1993, 49, 6773–6784. [Google Scholar] [CrossRef]
- Kalinin, V.N.; Shostakovskii, M.V.; Ponomarev, A.B. A new route to 2-aryl-4-quinolones via palladium-catalyzed carbonylative coupling of o-iodoanilines with terminal arylacetylenes. Tetrahedron Lett. 1992, 33, 373–376. [Google Scholar] [CrossRef]
- Haddad, N.; Tan, J.; Farina, V. Convergent synthesis of the quinolone substructure of BILN 2061 via carbonylative Sonogashira coupling/cyclization. J. Org. Chem. 2006, 71, 5031–5034. [Google Scholar] [CrossRef]
- Grimster, N.P.; Gauntlett, C.; Godfrey, C.R.A.; Gaunt, M.J. Palladium-catalyzed intermolecular alkenylation of indoles by solvent-controlled regioselective C-H functionalization. Angew. Chem. Int. Ed. Engl. 2005, 44, 3125–3129. [Google Scholar] [CrossRef]
- Battistuzzi, G.; Cacchi, S.; Fabrizi, G. The aminopalladation/reductive elimination domino reaction in the construction of functionalized Indole rings. Eur. J. Org. Chem. 2002, 2671–2681. [Google Scholar]
- Chouzier, S.; Gruber, M.; Djakovitch, L. New hetero-bimetallic Pd-Cu catalysts for the one-pot indole synthesis via the Sonogashira reaction. J. Mol. Catal. A: Chem. 2004, 212, 43–52. [Google Scholar] [CrossRef]
- Gruber, M.; Chouzier, S.; Koehler, K.; Djakovitch, L. Palladium on activated carbon: A valuable heterogeneous catalyst for one-pot multi-step synthesis. Appl. Catal. A: Gen. 2004, 265, 161–169. [Google Scholar] [CrossRef]
- Djakovitch, L.; Rollet, P. Sonogashira cross-coupling reactions catalysed by copper-free palladium zeolites. Adv. Synth. Catal. 2004, 346, 1782–1792. [Google Scholar] [CrossRef]
- Cacchi, S.; Fabrizi, G. Synthesis and functionalization of indoles through palladium-catalyzed reactions. Chem. Rev. 2005, 105, 2873–2920. [Google Scholar] [CrossRef]
- Djakovitch, L.; Dufaud, V.; Zaidi, R. Heterogeneous palladium catalysts applied to the synthesis of 2- and 2,3-functionalised indoles. Adv. Synth. Catal. 2006, 348, 715–724. [Google Scholar] [CrossRef]
- Djakovitch, L.; Rouge, P. New homogeneously and heterogeneously [Pd/Cu]-catalysed C3-alkenylation of free NH-indoles. J. Mol. Catal. A: Chem. 2007, 273, 230–239. [Google Scholar] [CrossRef]
- Djakovitch, L.; Rouge, P.; Zaidi, R. Selective arylation of 2-substituted indoles towards 1,2- and 2,3-functional indoles directed through the catalytic system. Catal. Commun. 2007, 8, 1561–1566. [Google Scholar] [CrossRef]
- Cusati, G.; Djakovitch, L. First heterogeneously palladium-catalysed fully selective C3-arylation of free NH-indoles. Tetrahedron Lett. 2008, 49, 2499–2502. [Google Scholar] [CrossRef]
- Djakovitch, L.; Rouge, P. Environmentally friendly [Pd/Cu]-catalysed C3-alkenylation of free NH-indoles. Catal. Today 2009, 140, 90–99. [Google Scholar]
- Noël, S.; Pinel, C.; Djakovitch, L. Direct synthesis of tricyclic 5H-pyrido[3,2,1-ij]quinolin-3-one by domino palladium catalyzed reaction. Org. Biol. Chem. 2006, 4, 3760–3762. [Google Scholar] [CrossRef]
- Rollet, P.; Kleist, W.; Dufaud, V.; Djakovitch, L. Copper-free heterogeneous catalysts for the Sonogashira cross-coupling reaction: Preparation, characterisation, activity and applications for organic synthesis. J. Mol Catal. A: Chem. 2005, 241, 39–51. [Google Scholar] [CrossRef]
- Larock, R.C.; Babu, S. Synthesis of nitrogen heterocycles via palladium-catalyzed intramolecular cyclization. Tetrahedron Lett. 1987, 28, 5291–5294. [Google Scholar] [CrossRef]
- Larock, R.C.; Yum, E.K. Synthesis of indoles via palladium-catalyzed heteroannulation of internal alkynes. J. Am. Chem. Soc. 1991, 113, 6689–6690. [Google Scholar] [CrossRef]
- Larock, R.C.; Yum, E.K.; Refvik, M.D. Synthesis of 2,3-disubstituted indoles via palladium-catalyzed annulation of internal alkynes. J. Org. Chem. 1998, 63, 7652–7662. [Google Scholar] [CrossRef]
- Huang, G.; Larock, R.C. Synthesis of substituted naphthalenes and carbazoles by the palladium-catalyzed annulation of internal alkynes. J. Org. Chem. 2003, 68, 7342–7349. [Google Scholar] [CrossRef]
- Braudeau, E.; David, S.; Fischer, J.C. 2-Hydroxyindoxyls. General and novel preparation, properties, and their role in the perphthalic acid oxidation of indoles. Tetrahedron 1974, 30, 1445–1455. [Google Scholar] [CrossRef]
- Hewitt, M.C.; Shao, L. Synthesis of 2-phenylindoxyls. ARKIVOC 2006, 37–46. [Google Scholar]
- Hooper, M.; Pitkethly, W.N. 2-(Arylmethylidene)-3-indolinones. Stereochemistry and reduction with sodium borohydride. J. Chem. Soc. Perkin Trans. 1 1972, 1607–1613. [Google Scholar]
- An, Z.W.; Catellani, M.; Chiusoli, G.P. A new palladium-catalyzed synthesis of indoxyl derivatives. J. Organomet. Chem. 1990, 397, C31–C32. [Google Scholar]
- The European Agency for the Evaluation of Medicinal Products, Evaluation of Medicines for Human Use. Available online: http://www.ema.europa.eu/ (accessed on 17 December 2002).
- In some cases, C2-alkenylation is observed. The C2/C3 selectivity could be oriented through the catalytic system and the reaction conditions.
- Higashijima, M.; Masunaga, T.; Kojima, Y.; Watanabe, E.; Wada, K. New hetero-bimetallic Pd-Cu catalyst for Wacker reaction. Stud. Surf. Sci. Catal. 1995, 92, 319–322. [Google Scholar]
- Gates, B.C. Catalytic Chemistry; John Wiley & Sons, Inc: NewYork, NY, USA, 1992; p. 259. [Google Scholar]
- Reddy, K.R.; Kumar, N.S.; Sreedhar, B.; Kantam, M.L. N-Arylation of nitrogen heterocycles with aryl halides and arylboronic acids catalyzed by cellulose supported copper(0). J. Mol Catal. A: Chem. 2006, 252, 136. [Google Scholar] [CrossRef]
- Kantam, M.L.; Rao, B.P.C.; Choudary, B.M.; Reddy, R.S. A mild and efficient method for N-arylation of nitrogen heterocycles with aryl halides catalyzed by Cu(II)-NaY zeolite. Synlett 2006, 2195–2198. [Google Scholar]
- Kantam, M.L.; Yadav, J.; Laha, S.; Sreedhar, B.; Jha, S. N-Arylation of heterocycles with activated chloro- and fluoroarenes using nanocrystalline copper(II) oxide. Adv. Synth. Catal. 2007, 349, 1938–1942. [Google Scholar] [CrossRef]
- Rout, L.; Jammi, S.; Punniyamurthy, T. Novel CuO nanoparticle catalyzed C-N cross coupling of amines with iodobenzene. Org. Lett. 2007, 9, 3397–3399. [Google Scholar] [CrossRef]
- Huang, Y.-Z.; Miao, H.; Zhang, Q.-H.; Chen, C.; Xu, J. Cu2O: A simple and efficient reusable catalyst for N-arylation of nitrogen-containing heterocycles with aryl halides. Catal. Lett. 2008, 122, 344–348. [Google Scholar] [CrossRef]
- Tang, B.-X.; Guo, S.-M.; Zhang, M.-B.; Li, J.-H. N-arylations of nitrogen-containing heterocycles with aryl and heteroaryl halides using a copper(I) oxide nanoparticle/1,10-phenanthroline catalytic system. Synthesis 2008, 1707–1716. [Google Scholar]
- Colacino, E.; Villebrun, L.; Martinez, J.; Lamaty, F. PEG3400-Cu2O-Cs2CO3: An efficient and recyclable microwave-enhanced catalytic system for ligand-free Ullmann arylation of indole and benzimidazole. Tetrahedron 2010, 66, 3730–3735. [Google Scholar] [CrossRef]
- Jadhav, V.H.; Dumbre, D.K.; Phapale, V.B.; Borate, H.B.; Wakharkar, R.D. Efficient N-arylation of amines catalyzed by Cu-Fe-hydrotalcite. Catal. Commun. 2006, 8, 65–68. [Google Scholar]
- Swapna, K.; Vijay, K.A.; Prakash, R.V.; Rama, R.K. Recyclable heterogeneous iron catalyst for C-N cross-coupling under ligand-free conditions. J. Org. Chem. 2009, 74, 7514–7517. [Google Scholar] [CrossRef]
- Chouhan, G.; Wang, D.; Alper, H. Magnetic nanoparticle-supported proline as a recyclable and recoverable ligand for the CuI catalyzed arylation of nitrogen nucleophiles. Chem. Commun. 2007, 4809–4811. [Google Scholar]
- Chen, W.; Zhang, Y.; Zhu, L.; Lan, J.; Xie, R.; You, J. A concept of supported amino acid ionic liquids and their application in metal scavenging and heterogeneous catalysis. J. Am. Chem. Soc. 2007, 129, 13879–13886. [Google Scholar] [CrossRef]
- Wang, L.; Yi, W.; Cai, C. Fluorous silica gel-supported perfluoro-tagged palladium nanoparticles: an efficient and reusable catalyst for direct C-2 arylation of indoles. Chem. Commun. 2011, 47, 806–808. [Google Scholar]
- Batail, N.; Bendjeriou, A.; Lomberget, T.; Barret, R.; Dufaud, V.; Djakovitch, L. First heterogeneous ligand- and salt-free larock indole synthesis. Adv. Synth. Catal. 2009, 351, 2055–2062. [Google Scholar] [CrossRef]
- Djakovitch, L.; Koehler, K. Heterogeneously catalysed Heck reaction using palladium modified zeolites. J. Mol Catal. A: Chem. 1999, 142, 275–284. [Google Scholar] [CrossRef]
- Djakovitch, L.; Koehler, K. Heck reaction catalyzed by Pd-Modified zeolites. J. Am. Chem. Soc. 2001, 123, 5990–5999. [Google Scholar] [CrossRef]
- Monguchi, Y.; Mori, S.; Aoyagi, S.; Tsutsui, A.; Maegawa, T.; Sajiki, H. Palladium on carbon-catalyzed synthesis of 2- and 2,3-substituted indoles under heterogeneous conditions. Org. Biol. Chem. 2010, 8, 3338–3342. [Google Scholar] [CrossRef]
- Batail, N.; Dufaud, V.; Djakovitch, L. Larock heteroannulation of 2-bromoanilines with internal alkynes via ligand and salt free Pd/C catalysed reaction. Tetrahedron Lett. 2011, 52, 1916–1918. [Google Scholar] [CrossRef]
- Shen, M.; Li, G.; Lu, B.Z.; Hossain, A.; Roschangar, F.; Farina, V.; Senanayake, C.H. The first regioselective palladium-catalyzed indolization of 2-bromo- or 2-chloroanilines with internal alkynes: A new approach to 2,3-disubstituted indoles. Org. Lett. 2004, 6, 4129–4132. [Google Scholar]
- Cui, X.; Li, J.; Fu, Y.; Liu, L.; Guo, Q.-X. Regioselective Pd-catalyzed indolization of 2-bromoanilines with internal alkynes using phosphine-free ligands. Tetrahedron Lett. 2008, 49, 3458–3462. [Google Scholar] [CrossRef]
- Batail, N.; Bendjeriou, A.; Djakovitch, L.; Dufaud, V. Larock indole synthesis using palladium complexes immobilized onto mesoporous silica. Appl. Catal. A: Gen. 2010, 388, 179–187. [Google Scholar] [CrossRef]
- Pal, M.; Subramanian, V.; Batchu, V.R.; Dager, I. Synthesis of 2-substituted indoles via Pd/C-catalyzed reaction in water. Synlett 2004, 1965–1969. [Google Scholar]
- Layek, M.; Lakshmi, U.; Kalita, D.; Barange, D.K.; Islam, A.; Mukkanti, K.; Pal, M. Pd/C-mediated synthesis of indoles in water. Beilstein J. Org. Chem. 2009, 5. No. 46. [Google Scholar]
- Djakovitch, L.; Rollet, P. Sonogashira cross-coupling reactions catalysed by heterogeneous copper-free Pd-zeolites. Tetrahedron Lett. 2004, 45, 1367–1370. [Google Scholar] [CrossRef]
- Hong, K.B.; Lee, C.W.; Yum, E.K. Synthesis of 2-substituted indoles by palladium-catalyzed heteroannulation with Pd-NaY zeolite catalysts. Tetrahedron Lett. 2004, 45, 693–697. [Google Scholar] [CrossRef]
- Nagamochi, M.; Fang, Y.Q.; Lautens, M. A general and practical method of alkynyl indole and benzofuran synthesis via tandem Cu- and Pd-catalyzed cross-couplings. Org. Lett. 2007, 9, 2955–2958. [Google Scholar] [CrossRef]
- Djakovitch, L.; Koehler, K.; De Vries, J.G. The role of palladium nanoparticles as catalysts for carbon-carbon coupling reactions. In Nanoparticles and Catalysis; Astruc, D., Ed.; Wiley-VCH: Weinheim, Germany, 2008; pp. 303–348. [Google Scholar]
- Djakovitch, L.; Wagner, M.; Hartung, C.G.; Beller, M.; Koehler, K. Pd-catalyzed Heck arylation of cycloalkenes-Studies on selectivity comparing homogeneous and heterogeneous catalysts. J. Mol. Catal. A: Chem. 2004, 219, 121–130. [Google Scholar] [CrossRef]
- Zhang, X.; Corma, A. Supported gold(III) catalysts for highly efficient three-component Coupling reactions. Angew. Chem. Int. Ed. 2008, 47, 4358–4361. [Google Scholar] [CrossRef]
- Felpin, F.-X.; Ibarguren, O.; Nassar-Hardy, L.; Fouquet, E. Synthesis of oxindoles by tandem Heck-Reduction-Cyclization (HRC) from a single bifunctional, in situ generated Pd/C catalyst. J. Org. Chem. 2009, 74, 1349–1352. [Google Scholar] [CrossRef]
- Felpin, F.-X.; Miqueu, K.; Sotiropoulos, J.-M.; Fouquet, E.; Ibarguren, O.; Laudien, J. Room-temperature, ligand- and base-free Heck Reactions of aryl diazonium salts at low palladium loading: Sustainable preparation of substituted stilbene derivatives. Chem. Eur. J. 2010, 16, 5191–5204. [Google Scholar] [CrossRef]
- Ibarguren, O.; Zakri, C.; Fouquet, E.; Felpin, F.-X. Heterogeneous palladium multi-task catalyst for sequential Heck-reduction-cyclization (HRC) reactions: Influence of the support. Tetrahedron Lett. 2009, 50, 5071–5074. [Google Scholar] [CrossRef]
- Felpin, F.-X.; Coste, J.; Zakri, C.; Fouquet, E. Preparation of 2-quinolones by sequential Heck Reduction-Cyclization (HRC) reactions by using a multitask palladium catalyst. Chem. Eur. J. 2009, 15, 7238–7245. [Google Scholar] [CrossRef]
- Leonard, N.J.; Herbrandson, H.F.; Vanheyningen, E.M. Synthesis of 4-hydroxyquinolines. 6. Synthesis of 3-normal-heptyl-7-methoxy-2-methyl-4-quinolinol. J. Am. Chem. Soc. 1946, 68, 1279–1281. [Google Scholar] [CrossRef]
- Chen, B.C.; Huang, X.; Wang, J. A versatile synthesis of 2-alkyl and 2-aryl 4-quinolones. Synthesis 1987, 482–484. [Google Scholar]
- Donnelly, J.A.; Farrell, D.F. The chemistry of 2'-amino analogs of 2'-hydroxychalcone and its derivatives. J. Org. Chem. 1990, 55, 1757–1761. [Google Scholar] [CrossRef]
- Kuo, S.C.; Lee, H.Z.; Juang, J.P.; Lin, Y.T.; Wu, T.S.; Chang, J.J.; Lednicer, D.; Paull, K.D.; Lin, C.M. Synthesis and cytotoxicity of 1,6,7,8-substituted 2-(4'-substituted phenyl)-4-quinolones and related compounds: identification as antimitotic agents interacting with tubulin. J. Med. Chem. 1993, 36, 1146–1156. [Google Scholar] [CrossRef]
- Birch, A.M.; Davies, R.V.; Maclean, L.; Robinson, K. Syntheses of flosequinan-A novel 4-quinolone shown to be useful in congestive-heart-failure. J. Chem. Soc. Perkin Trans. 1 1994, 387–392. [Google Scholar]
- Li, L.; Wang, H.-K.; Kuo, S.-C.; Wu, T.-S.; Lednicer, D.; Lin, C.M.; Hamel, E.; Lee, K.-H. Antitumor agents. 150. 2',3',4',5',5,6,7-Substituted 2-phenyl-4-quinolones and related compounds: Their synthesis, cytotoxicity, and inhibition of tubulin polymerization. J. Med. Chem. 1994, 37, 1126–1135. [Google Scholar] [CrossRef]
- Tollari, S.; Cenini, S.; Ragaini, F.; Cassar, L. Intramolecular amination of olefins-Synthesis of 2-substituted-4-quinolones from 2-nitrochalcones catalyzed by ruthenium. J. Chem. Soc. Chem. Commun. 1994, 1741–1742. [Google Scholar]
- Kayirere, M.-G.; Mahamoud, A.; Chevalier, J.; Soyfer, J.-C.; Crémieux, A.; Barbe, J. Synthesis and antibacterial activity of new 4-alkoxy, 4-aminoalkyl and 4-alkylthioquinoline derivatives. Eur. J. Med. Chem. 1998, 33, 55–63. [Google Scholar] [CrossRef]
- Wróbel, Z. Silane-mediated direct condensation of nitroarenes with cinnamyl-type sulfones. The way to 2-aryl-4-X-quinolines and their hetero analogs. Tetrahedron 1998, 54, 2607–2618. [Google Scholar] [CrossRef]
- Tollari, S.; Penoni, A.; Cenini, S. The unprecedented detection of the intermediate formation of N-hydroxy derivatives during the carbonylation of 2'-nitrochalcones and 2-nitrostyrenes catalysed by palladium. J. Mol. Catal. A. 2000, 152, 47–54. [Google Scholar] [CrossRef]
- Xia, Y.; Yang, Z.-Y.; Xia, P.; Hackl, T.; Hamel, E.; Mauger, A.; Wu, J.-H.; Lee, K.-H. Antitumor agents. 211. Fluorinated 2-phenyl-4-quinolone derivatives as antimitotic antitumor agents. J. Med. Chem. 2001, 44, 3932–3936. [Google Scholar] [CrossRef]
- Huang, X.; Liu, Z.X. Solid-phase synthesis of 4(1H)-quinolone and pyrimidine derivatives based on a new scaffold-polymer-bound cyclic malonic acid ester. J. Org. Chem. 2002, 67, 6731–6737. [Google Scholar] [CrossRef]
- Tang, J.; Huang, M. Preparation of resin-bound bismethylene cyclic malonic acid ester and facile solid-phase synthesis of 2-alkylthio-4(1H)-quinolone and 2-alkyl-4(1H)-quinolone. Synth. Commun. 2003, 33, 3953–3960. [Google Scholar] [CrossRef]
- Xia, Y.; Yang, Z.-Y.; Xia, P.; Bastow, K.F.; Nakanishi, Y.; Nampoothiri, P.; Hamel, E.; Brossi, A.; Lee, K.-H. Antitumor agents. Part 226: Synthesis and cytotoxicity of 2-phenyl-4-quinolone acetic acids and their esters. Bioorg. Med. Chem. Lett. 2003, 13, 2891–2893. [Google Scholar]
- Mphahlele, M.J.; El-Nahas, A.M. Tautomeric 2-arylquinolin-4(1H)-one derivatives-spectroscopic, X-ray and quantum chemical structural studies. J. Mol. Struct. 2004, 688, 129–136. [Google Scholar] [CrossRef]
- Huang, J.; Chen, Y.; King, A.O.; Dilmeghani, M.; Larsen, R.D.; Faul, M.M. A mild, one-pot synthesis of 4-quinolones via sequential Pd-catalyzed amidation and base-promoted cyclization. Org. Lett. 2008, 10, 2609–2612. [Google Scholar] [CrossRef]
- Bernini, R.; Cacchi, S.; Fabrizi, G.; Sferrazza, A. 1,2-Disubstituted 4-quinolones via copper-catalyzed cyclization of 1-(2-halophenyl)-2-en-3-amin-1-ones. Synthesis 2009, 1209–1219. [Google Scholar]
- Torii, S.; Okumoto, H.; Xu, L.H.; Sadakane, M.; Shostakovsky, M.V.; Ponomaryov, A.B.; Kalinin, V.N. Syntheses of chromones and quinolones via palladium-catalyzed carbonylation of o-iodophenols and anilines in the presence of acetylenes. Tetrahedron 1993, 49, 6773–6784. [Google Scholar] [CrossRef]
- Genelot, M.; Bendjeriou, A.; Dufaud, V.; Djakovitch, L. Optimised procedures for the one-pot selective syntheses of indoxyls and 4-quinolones by a carbonylative Sonogashira/cyclisation sequence. Appl. Catal. A: Gen. 2009, 369, 125–132. [Google Scholar] [CrossRef]
- Genelot, M.; Dufaud, V.; Djakovitch, L. Heterogeneous metallo-organocatalysis for the selective one-pot synthesis of 2-benzylidene-indoxyl and 2-phenyl-4-quinolone. Tetrahedron 2011, 67, 976–981. [Google Scholar] [CrossRef]
- Ambrogio, I.; Cacchi, S.; Fabrizi, G.; Prastaro, A. 3-(o-Trifluoroacetamidoaryl)-1-propargylic esters: common intermediates for the palladium-catalyzed synthesis of 2-aminomethyl-, 2-vinylic, and 2-alkylindoles. Tetrahedron 2009, 65, 8916–8929, [Note: In agreement with MS m/z (relative intensity): 207 (49), 206 (30), 130 (100), 102 (16), 77 (15). 1H-NMR (250 MHz, DMSO) δ 10.97 (s, 1H), 7.40 (d, J = 7.5 Hz, 1H), 7.33-7.11 (m, 6H), 6.95 (m, 2H), 6.13 (s, 1H), 4.05 (s, 2H)].. [Google Scholar] [CrossRef]
- Laudien, J.; Fouquet, E.; Zakri, C.; Felpin, F.-X. A multi-task palladium catalyst involved in Heck-Reduction-Cyclization sequences for the preparation of 4-benzyl-1,2-dihydroisoquinolin-3-ones: An unusual homogeneous-heterogeneous sustainable approach. Synlett 2010, 2010, 1539–1543. [Google Scholar] [CrossRef]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Djakovitch, L.; Batail, N.; Genelot, M. Recent Advances in the Synthesis of N-Containing Heteroaromatics via Heterogeneously Transition Metal Catalysed Cross-Coupling Reactions. Molecules 2011, 16, 5241-5267. https://doi.org/10.3390/molecules16065241
Djakovitch L, Batail N, Genelot M. Recent Advances in the Synthesis of N-Containing Heteroaromatics via Heterogeneously Transition Metal Catalysed Cross-Coupling Reactions. Molecules. 2011; 16(6):5241-5267. https://doi.org/10.3390/molecules16065241
Chicago/Turabian StyleDjakovitch, Laurent, Nelly Batail, and Marie Genelot. 2011. "Recent Advances in the Synthesis of N-Containing Heteroaromatics via Heterogeneously Transition Metal Catalysed Cross-Coupling Reactions" Molecules 16, no. 6: 5241-5267. https://doi.org/10.3390/molecules16065241