Induction of Apoptosis in Human Promyelocytic Leukemia HL60 Cells by Panaxynol and Panaxydol
Abstract
:1. Introduction
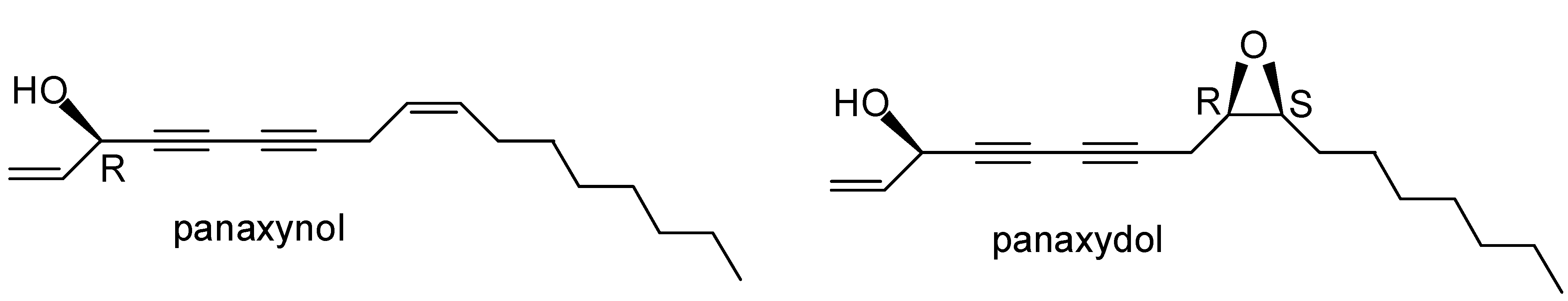
2. Results and Discussion
2.1. Effects of PNN and PND on Cell Growth

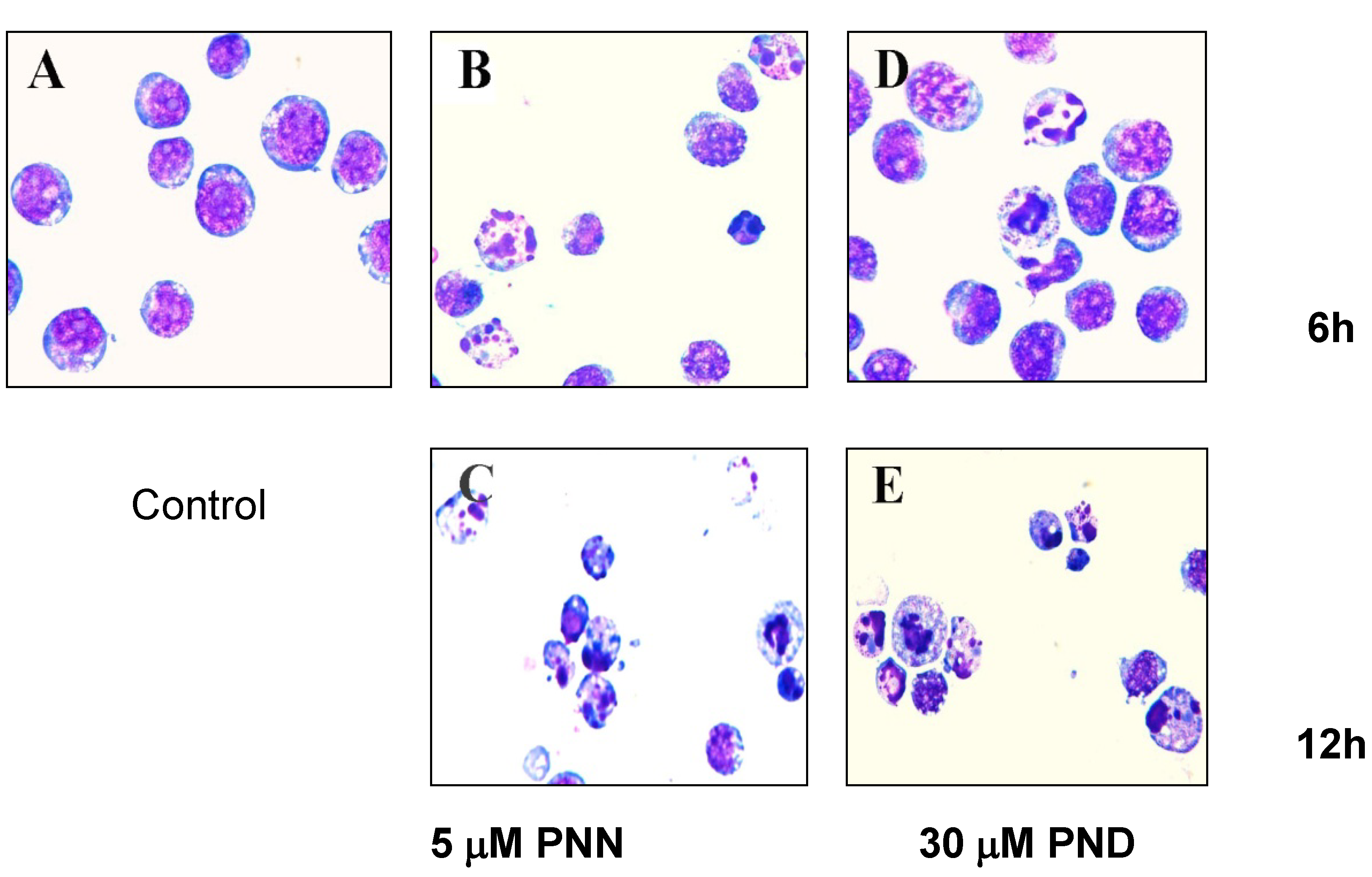
2.2. Morphological Analysis
2.3. Apoptosis Assays Using Flow Cytometer

2.4.Cell Cycle Analysis

2.5. Apoptosis-Related Proteins in HL60 Treated by PNN and PND

3. Experimental
3.1. General
3.2. Extraction and Isolation
3.3. PNN and PND Standards
3.4. Cell Culture and Drug Treatments
3.5. Cell Viability Assay
3.6. Morphological Analysis
3.7. Apoptosis Assays Using Flow Cytometer
3.8. Nuclear DNA Content Distribution
3.9. Western Blotting Analysis
3.10. Statistical Analysis
4. Conclusions
Acknowledgements
References and Notes
- Libura, J.; Slater, D.J.; Felix, C.A.; Richardson, C. Therapy-related acute myeloid leukemia-like MLL rearrangements are induced by etoposide in primary human CD34(+) cells and remain stable after clonal expansion. Blood 2005, 105, 2124–2131. [Google Scholar] [CrossRef]
- Mistry, A.R.; Felix, C.A.; Whitmarsh, R.J.; Mason, A.; Reiter, A.; Cassinat, B.; Parry, A.; Walz, C.; Wiemels, J.L.; Segal, M.R.; et al. DNA topoisomerase II in therapy-related acute promyelocytic leukemia. New Engl. J. Med. 2005, 352, 1529–1538. [Google Scholar]
- Nie, B.; Lu, Y.; Chen, Z. Progress in studies on naturally occurring polyacetylenes. Chin. Tradit. Herbal Drugs 2002, 33, 1050–1053. [Google Scholar]
- Christensen, L.P.; Brandt, K. Bioactive polyacetylenes in food plants of the Apiaceae family: Occurrence, bioactivity and analysis. J. Pharmaceut. Biomed. Anal. 2006, 41, 683–693. [Google Scholar] [CrossRef]
- Zidorn, C.; Johrer, K.; Ganzera, M.; Schubert, B.; Sigmund, E.M.; Mader, J.; Greil, R.; Ellmerer, E.P.; Stuppner, H. Polyacetylenes from the Apiaceae vegetables carrot, celery, fennel, parsley, and parsnip and their cytotoxic activities. J. Agr. Food Chem. 2005, 53, 2518–2523. [Google Scholar]
- Brandt, K.; Christensen, L.P.; Hansen-Møller, J.; Hansen, S.L.; Haraldsdottir, J.; Jespersen, L.; Purup, S.; Kharazmi, A.; Barkholt, V.; Frøkiær, H.; Kobæk-Larsen, M. Health promoting compounds in vegetables and fruits: A systematic approach for identifying plant components with impact on human health. Trends Food Sci. Technol. 2004, 15, 384–393. [Google Scholar] [CrossRef]
- Young, J.F.; Duthie, S.J.; Milne, L.; Christensen, L.P.; Duthie, G.G.; Bestwick, C.S. Biphasic effect of falcarinol on CaCo-2 cell proliferation, DNA damage, and apoptosis. J. Agr. Food Chem. 2007, 55, 618–623. [Google Scholar] [CrossRef]
- Siddiq, A.; Dembitsky, V. Acetylenic anticancer agents. Anti-Cancer Agents Med. Chem. 2008, 8, 132–170. [Google Scholar] [CrossRef]
- Guo, L.C.; Song, L.; Wang, Z.J.; Zhao, W.J.; Mao, W.W.; Yin, M. Panaxydol inhibits the proliferation and induces the differentiation of human hepatocarcinoma cell line HepG2. Chem.-Biol. Inter. 2009, 181, 138–143. [Google Scholar] [CrossRef]
- Kim, J.Y.; Yu, S.J.; Oh, H.J.; Lee, J.Y.; Kim, Y.; Sohn, J. Panaxydol induces apoptosis through an increased intracellular calcium level, activation of JNK and p38 MAPK and NADPH oxidase-dependent generation of reactive oxygen species. Apoptosis 2011, 16, 347–358. [Google Scholar] [CrossRef]
- Hai, J.; Lin, Q.; Lu, Y.; Zhang, H.; Yi, J. Induction of apoptosis in rat C6 glioma cells by panaxydol. Cell Biol. Int. 2007, 31, 711–715. [Google Scholar] [CrossRef]
- Wang, Z.J.; Wu, Y.L.; Lin, Q.; Chen, H.Z.; Lu, Y. Effect of panaxynol on differentiation of HL60 cells line in vitro induced. Chin. Tradit. Herbal Drugs 2003, 34, 736–738. [Google Scholar]
- Overbeeke, R.; Steffens-Nakken, H.; Vermes, I.; Reutelingsperger, C.; Haanen, C. Early features of apoptosis detected by four different flow cytometry assays. Apoptosis 1998, 3, 115–121. [Google Scholar] [CrossRef]
- Nicoletti, I.; Migliorati, G.; Pagliacci, M.C.; Grignani, F.; Riccardi, C. A rapid andsimple method for measuring thymocyte apoptosis by propidium iodide staining and flow-cytometry. J. Immunol. Methods 1991, 139, 271–279. [Google Scholar] [CrossRef]
- Nunez, G.; Benedict, M.A.; Hu, Y.M.; Inohara, N. Caspases: The proteases of the apoptotic pathway. Oncogene 1998, 17, 3237–3245. [Google Scholar]
- Wolf, B.B.; Schuler, M.; Echeverri, F.; Green, D.R. Caspase-3 is the primary activator of apoptotic DNA fragmentation via DNA fragmentation factor-45/inhibitor of caspase-activated DNase inactivation. J. Biol. Chem. 1999, 274, 30651–30656. [Google Scholar]
- Reyland, M.E. Protein kinase Cdelta and apoptosis. Biochem. Soc. Trans. 2007, 35, 1001–1004. [Google Scholar] [CrossRef]
- Humphries, M.J.; Limesand, K.H.; Schneider, J.C.; Nakayama, K.I.; Anderson, S.M.; Reyland, M.E. Suppression of apoptosis in the protein kinase Cdelta null mouse in vivo. J. Biol. Chem. 2006, 281, 9728–9737. [Google Scholar]
- Kato, K.; Yamanouchi, D.; Esbona, K.; Kamiya, K.; Zhang, F.; Kent, K.C.; Liu, B. Caspase-mediated protein kinase C-delta cleavage is necessary for apoptosis of vascular smooth muscle cells. Amer. J. Physiol. Heart Circ. Phy. 2009, 297, H2253–H2261. [Google Scholar] [CrossRef]
- Latchoumycandane, C.; Anantharam, V.; Kitazawa, M.; Yang, Y.; Kanthasamy, A.; Kanthasamy, A.G. Protein kinase Cdelta is a key downstream mediator of manganese-induced apoptosis in dopaminergic neuronal cells. J. Pharmacol. Exp. Ther. 2005, 313, 46–55. [Google Scholar]
- Day, T.W.; Wu, C.-H.; Safa, A.R. Etoposide Induces Protein Kinase Cδ- and Caspase-3-Dependent Apoptosis in Neuroblastoma Cancer Cells. Mol. Pharmacol. 2009, 76, 632–640. [Google Scholar] [CrossRef]
- Sun, F.; Kanthasamy, A.; Song, C.; Yang, Y.; Anantharam, V.; Kanthasamy, A.G. Proteasome inhibitor-induced apoptosis is mediated by positive feedback amplification of PKCdelta proteolytic activation and mitochondrial translocation. J. Cell. Mol. Med. 2008, 12, 2467–2481. [Google Scholar] [CrossRef]
- Song, M.G.; Gao, S.M.; Du, K.M.; Xu, M.; Yu, Y.; Zhou, Y.H.; Wang, Q.; Chen, Z.; Zhu, Y.S.; Chen, G.Q. Nanomolar concentration of NSC606985, a camptothecin analog, induces leukernic-cell apoptosis through protein kinase C delta-dependent mechanisms. Blood 2005, 105, 3714–3721. [Google Scholar] [CrossRef]
- Li, L.; Lorenzo, P.S.; Bogi, K.; Blumberg, P.M.; Yuspa, S.H. Protein kinase C delta targets mitochondria, alters mitochondrial membrane potential, and induces apoptosis in normal and neoplastic keratinocytes when overexpressed by an adenoviral vector. Mol. Cell Biol. 1999, 19, 8547–8558. [Google Scholar]
- Lin, Q.; Zhao, X.; Lu, Y.; Chen, Z.N. Study on the lipophilic components of Panax notoginseng. Chin. Tradit. Herbal Drugs 2002, 33, 490–492. [Google Scholar]
- Kobayashi, M.; Mahmud, T.; Umezome, T.; Wang, W.W.; Murakami, N.; Kitagawa, I. The absolute stereostructures of the polyacetylenic constituents of Ginseng Radix Rubra. Tetrahedron 1997, 53, 15691–15700. [Google Scholar]
- Kwon, B.M.; Ro, S.H.; Kim, M.K.; Nam, J.Y.; Jung, H.J.; Lee, I.R.; Kim, Y.K.; Bok, S.H. Polyacetylene analogs, isolated from hairy roots of Panax ginseng, inhibit Acyl-CoA: Cholesterol acyltransferase. Planta Med. 1997, 63, 552–553. [Google Scholar] [CrossRef]
- Lu, W.; Zheng, G.G.; Aisa, H.A.; Cai, J.C. First total synthesis of optically active panaxydol, a potential antitumor agent isolated from Panax ginseng. Tetrahedron Lett. 1998, 39, 9521–9522. [Google Scholar] [CrossRef]
- Zheng, G.R.; Lu, W.; Aisa, H.A.; Cai, J.C. Total synthesis of 3 (R)-panaxynol. Chin. Chem. Lett. 1998, 9, 1079–1080. [Google Scholar]
- Samples Availability: Samples of panaxynol and panaxydol are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yan, Z.; Yang, R.; Jiang, Y.; Yang, Z.; Yang, J.; Zhao, Q.; Lu, Y. Induction of Apoptosis in Human Promyelocytic Leukemia HL60 Cells by Panaxynol and Panaxydol. Molecules 2011, 16, 5561-5573. https://doi.org/10.3390/molecules16075561
Yan Z, Yang R, Jiang Y, Yang Z, Yang J, Zhao Q, Lu Y. Induction of Apoptosis in Human Promyelocytic Leukemia HL60 Cells by Panaxynol and Panaxydol. Molecules. 2011; 16(7):5561-5573. https://doi.org/10.3390/molecules16075561
Chicago/Turabian StyleYan, Zhonghong, Ruolin Yang, Yi Jiang, Zhihui Yang, Junrui Yang, Qian Zhao, and Yang Lu. 2011. "Induction of Apoptosis in Human Promyelocytic Leukemia HL60 Cells by Panaxynol and Panaxydol" Molecules 16, no. 7: 5561-5573. https://doi.org/10.3390/molecules16075561



