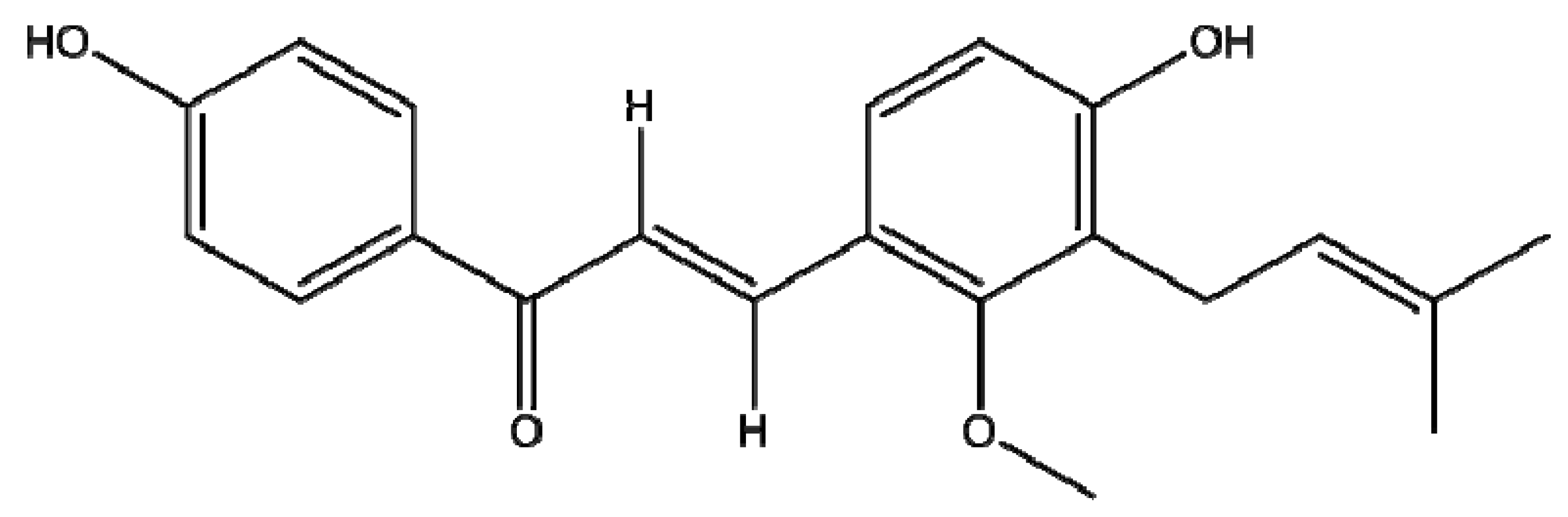
Licocalchone-C Extracted from Glycyrrhiza Glabra Inhibits Lipopolysaccharide-Interferon-γ Inflammation by Improving Antioxidant Conditions and Regulating Inducible Nitric Oxide Synthase Expression
Abstract
:1. Introduction
2. Results and Discussion
2.1. Cytotoxicities of Licochalcone C
2.2. Antioxidant Activity of Licochalcone C
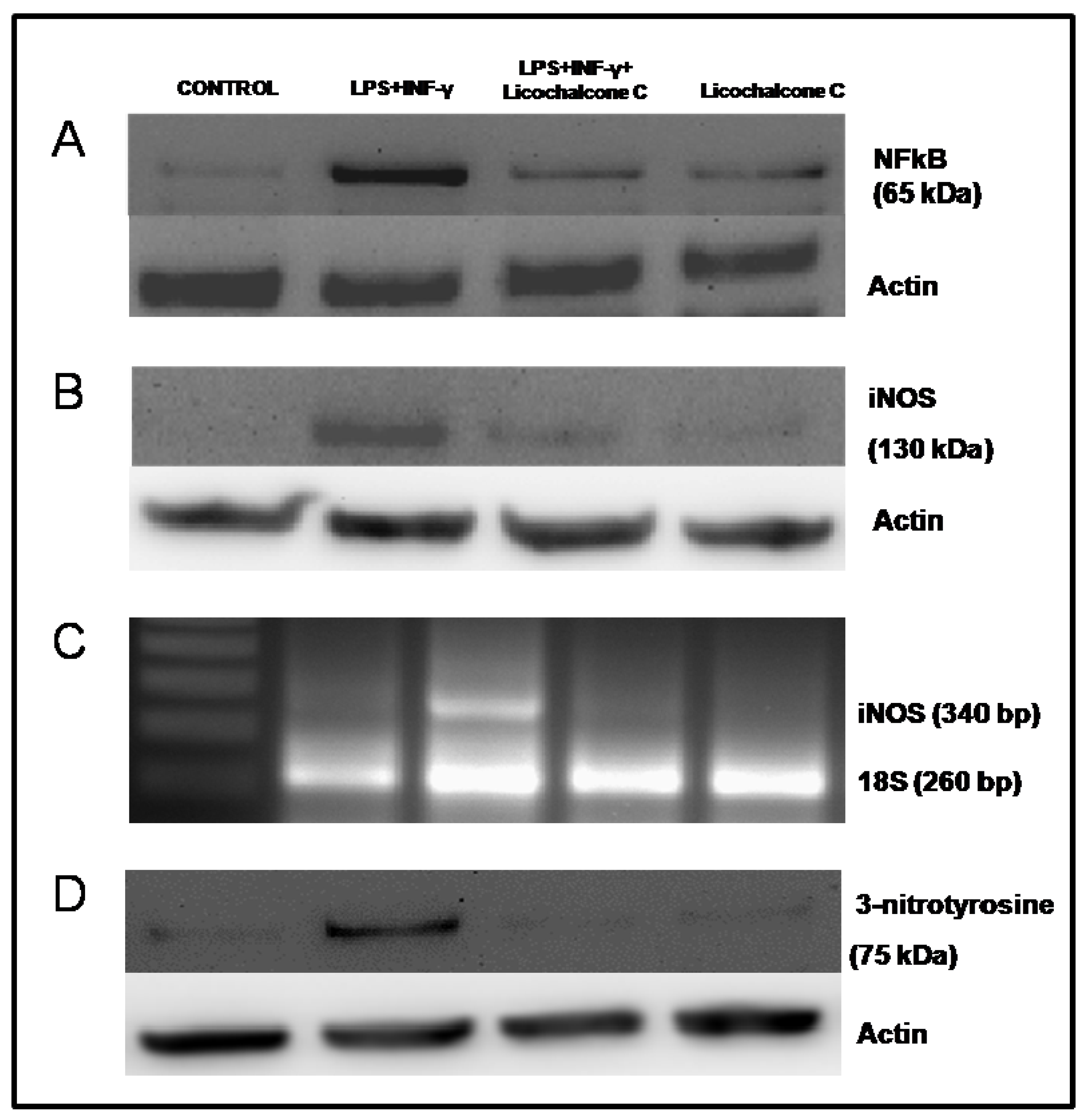
2.3. Influence of licochalcone C on iNOS signaling via NFkB
3. Experimental
3.1. General
3.2. Cell culture
3.3. Determination of O2−
3.4. Cu, Zn-Superoxide Dismutase Activity
3.5. Catalase Activity
3.6. Glutathione Peroxidase Activity
3.7. Protein Extraction and Isolation of Nuclei
3.8. Western blot Analysis for iNOS, NF-κB and 3-nitrotyrosine
3.9. Semi-Quantitative Reverse Transcription-Polymerase Chain Reaction for iNOS
3.10. Citrulline Synthesis
3.11. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of interest
References
- Asl, M.N.; Hosseinzadeh, H. Review of pharmacological effects of Glycyrrhiza sp. and its bioactive compounds. Phytother. Res. 2008, 22, 709–724. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Kaur, S.; Kumar, N.; Singh, B.; Kumar, S. Evaluation of antigenotoxic activity of isoliquiritin apioside from Glycyrrhiza glabra L. Toxicol. In Vitro 2009, 23, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Fiore, C.; Salvi, M.; Palermo, M.; Sinigagliab, G.; Armaninia, D.; Toninello, A. On the mechanism of mitochondrial permeability transition induction by glycyrrhetinic acid. Biochim. Biophys. Acta 2004, 1658, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Christensen, S.B.; Kharazmi, A. Antimalarial natural products. In Bioactive Compounds from Natural Sources: Isolation, Characterization and Biological Properties; Tringali, C., Ed.; Taylor and Francis Inc: New York, NY, USA, 2001. [Google Scholar]
- Deepak, M.; Setty, M.; D’Souza, P.; Agarwal, A.; Sangli, G.K. Bioactive caffeic acid esters from Glycyrrhiza glabra. Nat. Prod. Res. 2009, 23, 1657–1663. [Google Scholar]
- Belinky, P.A.; Aviram, M.; Furhman, B.; Rosenblat, M.; Vaya, J.A. The antioxidative effects of the isoflavan glabridin on endogenous constituents of LDL during its oxidation. Atherosclerosis 1998, 137, 49–61. [Google Scholar] [CrossRef]
- Li, W.; Asada, Y.; Yoshikawa, T. Flavonoid constituents from Glycyrrhiza glabra hairy root cultures. Phytochemistry 2000, 55, 447–456. [Google Scholar] [CrossRef]
- Hollman, P.C.H. Bioavailability of flavonoids. Eur. J. Clin. Nutr. 1997, 51, S66–S69. [Google Scholar] [PubMed]
- Davis, J.M.; Murphy, E.A.; Carmichael, M.D. Effects of the dietary flavonoid quercetin upon performance and health. Curr. Sports Med. Rep. 2009, 8, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Benavente-García, O.; Castillo, J. Update on uses and properties of citrus flavonoids: New findings in anticancer, cardiovascular, and anti-inflammatory activity. J. Agric. Food Chem. 2008, 56, 6185–6205. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, C.; Batra, S.; Vargo, M.A.; Voss, O.H.; Gavrilin, M.A.; Wewers, M.D.; Guttridge, D.C.; Grotewold, E.; Doseff, A.I. Apigenin blocks lipopolysaccharide-induced lethality in vivo and proinflammatory cytokines expression by inactivating NF-kappaB through the suppression of p65 phosphorylation. J. Immunol. 2007, 179, 7121–7127. [Google Scholar] [CrossRef] [PubMed]
- Bremner, P.; Heinrich, M. Natural products as targeted modulators of the nuclear factor-kappaB pathway. J. Pharm. Pharmacol. 2002, 54, 453–472. [Google Scholar] [CrossRef] [PubMed]
- Tsukahara, M.; Nishino, T.; Furuhashi, I.; Inoue, H.; Sato, T.; Matsumoto, H. Synthesis and inhibitory effect of novel glycyrrhetinic acid derivatives on IL-1b-induced prostaglandin E2 production in normal human dermal fibroblasts. Chem. Pharm. Bull. 2005, 53, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, V.; Karin, M. Regulation and Function of NF-κB Transcription Factors in the Immune System. Annu. Rev. Immunol. 2009, 27, 693–733. [Google Scholar]
- Korhonen, R.; Lahti, A.; Kankaanranta, H.; Moilanen, E. Nitric oxide production and signaling in inflammation. Curr. Drug Target. Inflam. Allergy 2005, 4, 471–479. [Google Scholar] [CrossRef]
- Zamora, R.; Vodovotz, Y.; Billiar, T.R. Inducible Nitric Oxide Synthase and Inflammatory Diseases. Mol. Med. 2000, 6, 347–373. [Google Scholar] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Ryter, S.W.; Kim, H.P.; Hoetzel, A.; Park, J.W.; Nakahira, K.; Wang, X.; Choi, A.M. Mechanisms of cell death in oxidative stress. Antioxid. Redox Signal 2007, 9, 49–89. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, G.; Moshage, H.; van Dullemen, H.M.; de Jager-Krikken, A.; Tiebosch, A.T.; Kleibeuker, J.H.; Jansen, P.L.; van Goor, H. Expression of nitric oxide sinthases and formation of nitrotyrosine and reactive oxygene species in inflammatory bowel disease. J. Pathol. 1998, 186, 416–421. [Google Scholar] [CrossRef]
- Haraguchi, H.; Ishikawa, H.; Mizutani, K.; Tamura, Y.; Kinoshita, T. Antioxidative and superoxide scavenging activities of retrochalcones in Glycyrrhiza inflata. Bioorg. Med. Chem. 1998, 6, 339–347. [Google Scholar] [CrossRef]
- Chang, H.J.; Yoon, G.; Park, J.S.; Kim, M.H.; Baek, M.K.; Kim, N.H.; Shin, B.A.; Ahn, B.W.; Cheon, S.H.; Jung, Y.D. Induction of apoptosis by the licochalcone E in endothelial cells via modulation of NF-kappaB and Bcl-2 Family. Biol. Pharm. Bull. 2007, 30, 2290–2293. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Speranza, L.; Franceschelli, S.; Pesce, M.; Reale, M.; Menghini, L.; Vinciguerra, I.; De Lutiis, M.A.; Felaco, M.; Grilli, A. The antiinflammatory action in THP-1 cells treated with verbasco side. Phytother. Res. 2010, 24, 1398–1404. [Google Scholar] [CrossRef] [PubMed]
- Dobashi, K.; Pahan, K.; Chahal, A.; Singh, I. Modulation of endogenous antioxidant enzymes by nitric oxide in rat C6 glial cells. J. Neurochem. 1997, 68, 1896–1903. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.C. Reversible binding and inhibition of catalase by nitric oxide. Eur. J. Biochem. 1995, 232, 188–191. [Google Scholar] [CrossRef] [PubMed]
- Hertog, M.G.L.; Hollman, P.C.H. Potential health effects of the dietary flavonol quercetin. Eur. J. Clin. Nutr. 1996, 50, 63–71. [Google Scholar]
- Reale, M.; De Lutiis, M.A.; Patruno, A.; Speranza, L.; Felaco, M.; Grilli, A.; Macrì, M.A.; Comani, S.; Conti, P.; Di Luzio, S. Modulation of MCP-1 and iNOS by 50-Hz sinusoidal electromagnetic field. Nitric Oxide 2006, 15, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Madonna, R.; Di Napoli, P.; Massaro, M.; Grilli, A.; Felaco, M.; De Caterina, A.; Tang, D.; De Caterina, R.; Geng, Y.J. Simvastatin attenuates expression of cytokine-inducible nitric-oxide synthase in embryonic cardiac myoblasts. J. Biol. Chem. 2005, 280, 13503–13511. [Google Scholar] [CrossRef] [PubMed]
- Speranza, L.; Franceschelli, S.; Pesce, M.; Vinciguerra, I.; De Lutiis, M.A.; Grilli, A.; Felaco, M.; Patruno, A. Phosphodiesterase type-5 inhibitor and oxidative stress. Int. J. Immunopathol. Pharmacol. 2008, 21, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Iarlori, C.; Gambi, D.; Lugaresi, A.; Patruno, A.; Felaco, M.; Salvatore, M.; Speranza, L.; Reale, M. Reduction of free radicals in multiple sclerosis: effect of glatiramer acetate (Copaxone). Mult. Scler. 2008, 14, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Conforti, F.; Menichini, F. Phenolic compounds from plants as nitric oxide production inhibitors. Curr. Med. Chem. 2011, 18, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Yoon, G.; Kang, B.Y.; Cheon, S.H. Topoisomerase I inhibition and cytotoxicity of licochalcones A and E from Glycyrrhiza inflata. Arch. Pharm. Res. 2007, 30, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, K.A.; Groszek, L.; Smalley, D.M.; Sessa, W.C.; Wu, M.; Villalon, P.; Wolin, M.S.; Stemerman, M.B. Native low-density lipoprotein increases endothelial cell nitric oxide synthase generation of superoxide anion. Circ. Res. 1995, 77, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Zigman, S. An improved spectrophotometric assay for superoxide dismutase based on epinephrine autoxidation. Anal. Biochem. 1978, 90, 81–89. [Google Scholar] [CrossRef]
- Aebi, H.E. Catalase. In Methods in Enzymatic Analysis; Bergmeyer, H.U., Ed.; Verlag Chemie: Weinheim, Germany, 1974; pp. 673–684. [Google Scholar]
- Paglia, D.E.; Valentine, W.N. Studies on the qualitative and quantitative characterization of erythrocytes glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar] [PubMed]
- Di Ilio, C.; Sacchetta, P.; Lo Bello, M.; Caccuri, G.; Federici, G. Selenium independent glutathione peroxidase activity associated with cationic forms of glutathione transferase in human heart. J. Mol. Cell Cardiol. 1986, 18, 983–991. [Google Scholar] [CrossRef]
- Armanini, D.; Fiore, C.; Mattarello, M.J.; Bielenberg, J.; Palermo, M. History of the endocrine effects of licorice. Exp. Clin. Endocrinol. Diabetes 2002, 110, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Shibata, S.; Inoue, H.; Iwata, S.; Ma, R.D.; Yu, L.J.; Ueyama, H.; Takayasu, J.; Hasegawa, T.; Tokuda, H.; Nishino, A. Inhibitory effects of licochalcone A isolated from Glycyrrhiza inflata root on inflammatory ear edema and tumour promotion in mice. Planta Med. 1991, 57, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Nixon, D.W. Licorice and cancer. Nutr. Cancer 2001, 39, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Shibata, S. A drug over the millennia: Pharmacognosy, chemistry, and pharmacology of licorice. Yakugaku Zasshi 2000, 120, 849–862. [Google Scholar] [CrossRef] [PubMed]
- Wittschier, N.; Faller, G.; Hensel, A. Aqueous extracts and polysaccharides from liquorice roots (Glycyrrhiza glabra L.) inhibit adhesion of Helicobacter pylori to human gastric mucosa. J. Ethnopharmacol. 2009, 125, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.C.; Dong, X.W.; Wu, X.M.; Yan, X.F.; Xie, Q.M. Inhibitory effects of flavonoids extracted from licorice on lipopolysaccharide-induced acute pulmonary inflammation in mice. Int. Immunopharmacol. 2009, 9, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Kao, T.C.; Shyu, M.H.; Yen, G.C. Glycyrrhizic acid and 18beta-glycyrrhetinic acid inhibit inflammation via PI3K/Akt/GSK3beta signaling and glucocorticoid receptor activation. J. Agric. Food Chem. 2010, 58, 8623–8629. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compound are available from the authors. |



| Before incubation | After 24 hr of incubation | ||
|---|---|---|---|
| Control cells | 96.2% | 95% | |
| Cells + 500 μM Licochalcone C | 91% | p < 0.05 | |
| Cells + 100 μM Licochalcone C | 91.4% | p < 0.05 | |
| Cells + 50 μM Licochalcone C | 94.4% | p < 0.05 |
| CONTROL | LPS + INF-γ | LPS + INF-γ + Licochalcone C | Licochalcone C | |
|---|---|---|---|---|
| NFkB Western Blotting | 0.23 ± 0.04 | 1.17 ± 0.04 | 0.39 ± 0.03* | 0.31 ± 0.03 |
| iNOS Western Blotting | 0.17 ± 0.05 | 1.01 ± 0.03 | 0.28 ± 0.04* | 0.23 ± 0.03 |
| iNOS rt-PCR | 0.25 ± 0.04 | 0.62 ± 0.04 | 0.15 ± 0.02* | 0.16 ± 0.03 |
| 3-Nitrotyrosine Western Blotting | 0.23 ± 0.06 | 1.12 ± 0.02 | 0.27 ± 0.05* | 0.17 ± 0.04 |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Franceschelli, S.; Pesce, M.; Vinciguerra, I.; Ferrone, A.; Riccioni, G.; Antonia, P.; Grilli, A.; Felaco, M.; Speranza, L. Licocalchone-C Extracted from Glycyrrhiza Glabra Inhibits Lipopolysaccharide-Interferon-γ Inflammation by Improving Antioxidant Conditions and Regulating Inducible Nitric Oxide Synthase Expression. Molecules 2011, 16, 5720-5734. https://doi.org/10.3390/molecules16075720
Franceschelli S, Pesce M, Vinciguerra I, Ferrone A, Riccioni G, Antonia P, Grilli A, Felaco M, Speranza L. Licocalchone-C Extracted from Glycyrrhiza Glabra Inhibits Lipopolysaccharide-Interferon-γ Inflammation by Improving Antioxidant Conditions and Regulating Inducible Nitric Oxide Synthase Expression. Molecules. 2011; 16(7):5720-5734. https://doi.org/10.3390/molecules16075720
Chicago/Turabian StyleFranceschelli, Sara, Mirko Pesce, Isabella Vinciguerra, Alessio Ferrone, Graziano Riccioni, Patruno Antonia, Alfredo Grilli, Mario Felaco, and Lorenza Speranza. 2011. "Licocalchone-C Extracted from Glycyrrhiza Glabra Inhibits Lipopolysaccharide-Interferon-γ Inflammation by Improving Antioxidant Conditions and Regulating Inducible Nitric Oxide Synthase Expression" Molecules 16, no. 7: 5720-5734. https://doi.org/10.3390/molecules16075720






