Synthesis and Biological Evaluation of Novel 99mTc-Labelled Bisphosphonates as Superior Bone Imaging Agents
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry and Radiolabeling
2.2. In Vitro Stability and Octanol-Water Partition Coefficient
2.3. Plasma Protein Binding
2.4. Blood Kinetics Studies
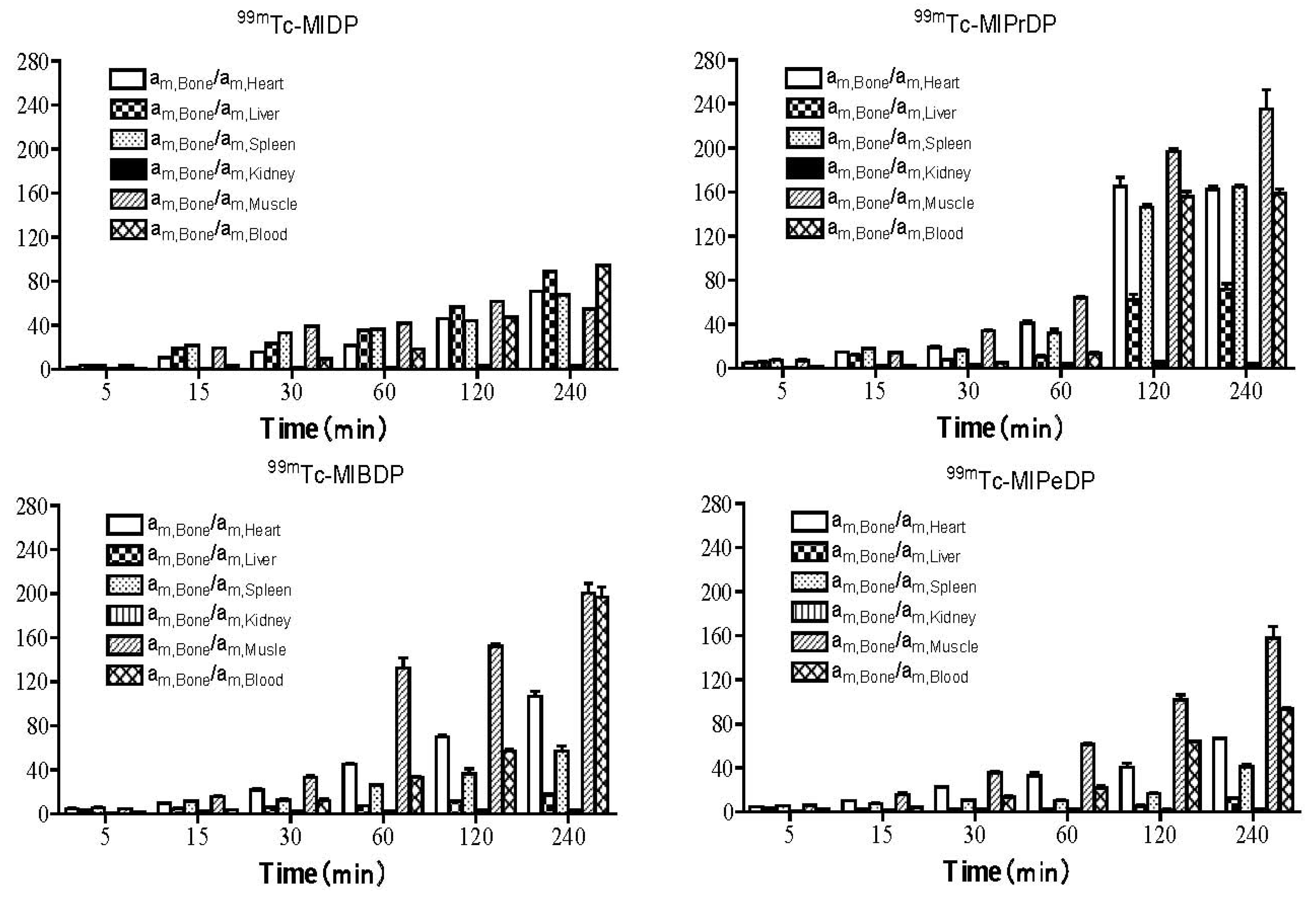
2.5. Biodistribution Studies
3. Experimental
3.1. General
3.2. Syntheses of Diphosphonic Acids
3.2.1. General Procedure for the Preparation of Compounds 3a-3c
3.2.2. General Procedure for the Preparation of Compound 4
3.3. Radiochemical Syntheses of 5a-5c
3.4. Quality Control of 5a-5c
3.4.1. TLC
3.4.2. HPLC
3.5. In Vitro Stability of 5a-5c
3.6. Octanol-Water Partition Coefficients of 5a-5c
3.7. Plasma protein Binding Assay
3.8. In Vivo Distribution of 5a-5c
3.9. Blood Kinetics Studies
4. Conclusions
Acknowledgment
References
- King, M.A.; Weber, D.A.; Casarett, G.W.; Burgener, F.A.; Corriveau, O. A study of irradiated bone. Part II: Changes in Tc-99m pyrophosphate bone imaging. J. Nucl. Med. 1980, 21, 22–30. [Google Scholar] [PubMed]
- Valdez, V.A.; Jacobstein, J.G. Decreased bone uptake of technetium-99m polyphosphate inthalassemia major. J. Nucl. Med. 1980, 21, 47–49. [Google Scholar] [PubMed]
- Davis, M.A.; Jones, A.G. Comparison of 99mTc-labeled phosphate and phosphonate agents for skeletal imaging. J. Nucl. Med. 1976, 6, 19–31. [Google Scholar] [CrossRef]
- Subramanian, G.; McAfee, J.G.; Blair, R.J. Technetium-99m-methylene diphosphonate-a superior agent for skeletal imaging: Comparison with other technetium complexes. J. Nucl. Med. 1975, 16, 744–755. [Google Scholar] [PubMed]
- Fogelman, I.; Pearson, D.W.; Bessent, R.G.; Tofe, A.J.; Francis, M.D. A comparison of skeletal uptakes of three diphosphonates by whole-body. J. Nucl. Med. 1981, 22, 880–883. [Google Scholar] [PubMed]
- Cole, T.J.; Balseiro, J.; Lippman, H.R. Technetium-99m-methylene diphosphonate (MDP) uptake in a sympathetic effusion: An index of malignancy and a review of the literature. J. Nucl. Med. 1991, 32, 325–327. [Google Scholar] [PubMed]
- Shalaby-Rana, E.; Majd, M. 99mTc-MDP scintigraphic findings in children with leukemia: Value of early and delayed whole-body imaging. J. Nucl. Med. 2001, 42, 878–883. [Google Scholar] [PubMed]
- Love, C.; Din, A.S.; Tomas, M.B.; Kalapparambath, T.P.; Palestro, C.J. Radionuclide bone imaging: An illustrative review. Radiographics 2003, 23, 341–358. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Mukai, T.; Inoue, Y.; Ono, M.; Saji, H. Development of a novel 99mTc-chelate conjugated bisphosphonate with high affinity for bone as a bone scintigraphic agent. J. Nucl. Med. 2006, 47, 2042–2047. [Google Scholar] [PubMed]
- Smith, M.R. Osteoclast targeted therapy for prostate cancer: Bisphosphonates and beyond. Urol. Oncol.: Semin. Orig. Investig. 2008, 26, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Luo, S.N.; Xie, M.H.; Liu, X.Y.; Feng, Y.Y.; Chen, Z.M. The new bone imaging agent: Preparation and biodistribution of 99Tcm-ZL. Nucl. Tech. (in Chinese) 2006, 29, 438–441. [Google Scholar]
- Niu, G.S.; Luo, S.N.; Yan, X.H.; Yang, M.; Ye, W.Z.; Wang, H.Y. The preparation and biodistribution of 99Tcm-EIDP. Nucl. Tech. (in Chinese) 2008, 31, 698–701. [Google Scholar]
- Chen, C.Q.; Luo, S.N.; Lin, J.G.; Yang, M.; Ye, W.Z.; Qiu, L. Preparation and biodistribution of 99Tcm-PIDP as bone imaging agent. Nucl. Sci. Tech. 2009, 20, 302–306. [Google Scholar]
- Lin, J.G.; Luo, S.N.; Chen, C.Q.; Qiu, L.; Wang, Y.; Cheng, W. Preparation and preclinical pharmacological study on a novel bone imaging agent 99mTc-EMIDP. Appl. Radiat. Isot. 2010, 9, 1616–1622. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Luo, S.N.; Lin, J.G.; Qiu, L.; Cheng, W.; Zhai, H.Z.; Nan, B.B.; Ye, W.Z.; Xia, Y.Y. Animal studies of 99mTc-i-PIDP: A new bone imaging agent. Appl. Radiat. Isot. 2011, 69, 1169–1175. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.G.; Qiu, L.; Cheng, W.; Luo, S.N.; Ye, W.Z. Preparation and in vivo biological investigations on a novel radioligand for bone scanning: Technetium-99m-labeled zoledronic acid derivative. Nuel. Med. Biol. 2011, 38, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Verbeke, K.; Rozenski, J.; Cleynhens, B.; Vanbilloen, H.; Groot, T.; Weyns, N.; Bormans, G.; Verbruggen, A. Development of a conjugate of 99mTc-EC with aminomethylene diphosphonate in the search for a bone tracer with fast clearance from soft Tissue. Bioconjug. Chem. 2002, 13, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Valko, K. Application of high-performance liquid chromatography based measurements of lipophilicity to model biological distribution. J. Chromatogr. A 2004, 1037, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Kroesbergen, J.; Roozen, A.M.P.; Wortelboer, M.R.; Gelsema, W.J.; DeLigny, C.L. 99mTc bone scanning agents-VI. Gel chromatographic analysis of the plasma protein binding of 99mTc(Sn)pyrophosphate, 99mTc(Sn)MDP and 99mTc(Sn)HMDP. Nucl. Med. Biol. 1988, 5, 479–487. [Google Scholar] [CrossRef]
- Vallner, J.J. Binding of drugs by albumin and plasma protein. J. Pharm. Sci. 1977, 66, 447–465. [Google Scholar] [CrossRef] [PubMed]
- Major, P.; Lortholary, A.; Hon, J.; Abdi, E.; Mills, G.; Menssen, H.D.; Yunus, F.; Bell, R.; Body, J.; Quebe-Fehling, E.; Seaman, J. Zoledronic acid is superior to pamidronate in the treatment of hypercalcemia of malignancy: A pooled analysis of two randomized, controlled clinical trials. J. Clin. Oncol. 2001, 19, 558–567. [Google Scholar] [CrossRef] [PubMed]
- Berenson, J.R. Recommendations for zoledronic acid treatment of patients with bone metastases. Oncologist 2005, 10, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Widler, L.; Jaeggi, K.A.; Glatt, M.; Müller, K.; Bachmann, R.; Bisping, M.; Born, A.R.; Cortesi, R.; Guiglia, G.; Jeker, H.; et al. Highly potent geminal bisphosphonates. From pamidronate disodium (aredia) to zoledronic acid (zometa). J. Med. Chem. 2002, 45, 3721–3738. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |




| Constituent | pH = 7.0 | pH = 7.4 |
|---|---|---|
| 99mTc-MIPrDP | −1.89 ± 0.05 | −1.71 ± 0.12 |
| 99mTc-MIBDP | −1.93 ± 0.09 | −1.77 ± 0.05 |
| 99mTc-MIPeDP | −2.10 ± 0.07 | −1.89 ± 0.07 |
| Parameters | 99mTc-MIPrDP | 99mTc-MIBDP | 99mTc-MIPeDP |
|---|---|---|---|
| K12 (min−1) | 0.0266 | 0.0286 | 0.0873 |
| K21 (min−1) | 0.0209 | 0.0087 | 0.0493 |
| Ke (min−1) | 0.0193 | 0.0221 | 0.0369 |
| CL (%ID/g/min) | 2.5887 | 2.9035 | 3.8432 |
| T1/2α (min) | 11.519 | 12.369 | 4.269 |
| T1/2β (min) | 102.78 | 202.12 | 61.64 |
| AUC (%ID/g * min) | 142.92 | 127.43 | 96.21 |
| Tissue | Time after injection | |||||
|---|---|---|---|---|---|---|
| 5 min | 15 min | 30 min | 60 min | 120 min | 240 min | |
| 99mTc-MIDP [11] | ||||||
| Heart | 3.03 ± 0.18 | 1.23 ± 0.05 | 0.84 ± 0.01 | 0.54 ± 0.02 | 0.31 ± 0.02 | 0.20 ± 0.01 |
| Liver | 1.52 ± 0.03 | 0.68 ± 0.02 | 0.56 ± 0.03 | 0.33 ± 0.03 | 0.25 ± 0.01 | 0.16 ± 0.01 |
| Spleen | 1.73 ± 0.01 | 0.60 ± 0.01 | 0.40 ± 0.02 | 0.32 ± 0.04 | 0.32 ± 0.01 | 0.21 ± 0.02 |
| Kidney | 19.7 ± 1.22 | 12.1 ± 2.11 | 8.42 ± 0.22 | 7.82 ± 0.88 | 4.54 ± 0.52 | 4.14 ± 0.67 |
| Muscle | 1.65 ± 0.34 | 0.66 ± 0.02 | 0.34 ± 0.01 | 0.28 ± 0.01 | 0.23 ± 0.02 | 0.26 ± 0.03 |
| Blood | 8.54 ± 0.92 | 3.90 ± 0.11 | 1.35 ± 0.22 | 0.63 ± 0.02 | 0.30 ± 0.01 | 0.15 ± 0.01 |
| All bone | 11.1 ± 0.08 | 12.8 ± 0.23 | 13.2 ± 1.11 | 11.6 ± 1.22 | 14.2 ± 0.45 | 14.2 ± 0.23 |
| 99mTc-MIPrDP | ||||||
| Heart | 1.41 ± 0.03 | 0.71 ± 0.07 | 0.53 ± 0.02 | 0.30 ± 0.01 | 0.12 ± 0.01 | 0.07 ± 0.00 |
| Liver | 1.17 ± 0.03 | 0.87 ± 0.06 | 1.35 ± 0.09 | 1.15 ± 0.02 | 0.32 ± 0.05 | 0.16 ± 0.00 |
| Spleen | 0.86 ± 0.03 | 0.59 ± 0.03 | 0.64 ± 0.01 | 0.38 ± 0.02 | 0.14 ± 0.00 | 0.07 ± 0.00 |
| Kidney | 8.35 ± 0.11 | 4.58 ± 0.19 | 3.44 ± 0.23 | 2.54 ± 0.05 | 3.21 ± 0.50 | 2.78 ± 0.09 |
| Muscle | 0.86 ± 0.02 | 0.58 ± 0.11 | 0.34 ± 0.03 | 0.19 ± 0.00 | 0.10 ± 0.01 | 0.05 ± 0.00 |
| Blood | 5.00 ± 0.02 | 2.69 ± 0.19 | 1.54 ± 0.12 | 0.74 ± 0.05 | 0.13 ± 0.01 | 0.08 ± 0.00 |
| All bone | 6.43 ± 0.40 | 10.5 ± 0.12 | 10.2 ± 0.56 | 12.2 ± 0.05 | 19.6 ± 0.87 | 11.5 ± 0.27 |
| 99mTc-MIBDP | ||||||
| Heart | 1.43 ± 0.04 | 1.02 ± 0.08 | 0.63 ± 0.03 | 0.36 ± 0.02 | 0.16 ± 0.00 | 0.11 ± 0.01 |
| Liver | 1.85 ± 0.08 | 2.96 ± 0.07 | 3.59 ± 0.25 | 1.79 ± 0.12 | 1.14 ± 0.04 | 0.81 ± 0.01 |
| Spleen | 1.12 ± 0.03 | 1.05 ± 0.04 | 0.95 ± 0.03 | 0.52 ± 0.03 | 0.41 ± 0.03 | 0.29 ± 0.01 |
| Kidney | 8.36 ± 0.28 | 6.53 ± 0.33 | 5.62 ± 0.46 | 5.21 ± 0.40 | 4.45 ± 0.22 | 5.78 ± 0.20 |
| Muscle | 0.87 ± 0.08 | 0.55 ± 0.11 | 0.41 ± 0.03 | 0.15 ± 0.00 | 0.08 ± 0.00 | 0.05 ± 0.00 |
| Blood | 3.79 ± 0.07 | 1.54 ± 0.06 | 0.81 ± 0.00 | 0.35 ± 0.02 | 0.19 ± 0.00 | 0.09 ± 0.00 |
| All bone | 6.56 ± 0.20 | 11.1 ± 1.45 | 12.9 ± 0.42 | 17.1 ± 1.08 | 11.2 ± 0.13 | 16.8 ± 0.88 |
| 99mTc-MIPeDP | ||||||
| Heart | 1.83 ± 0.05 | 0.99 ± 0.03 | 0.52 ± 0.02 | 0.39 ± 0.02 | 0.32 ± 0.03 | 0.16 ± 0.00 |
| Liver | 1.89 ± 0.04 | 5.31 ± 0.33 | 4.58 ± 0.16 | 4.20 ± 0.13 | 2.25 ± 0.24 | 0.90 ± 0.01 |
| Spleen | 1.07 ± 0.04 | 1.80 ± 0.14 | 1.45 ± 0.04 | 1.27 ± 0.03 | 0.67 ± 0.02 | 0.28 ± 0.02 |
| Kidney | 8.15 ± 0.26 | 7.05 ± 0.24 | 6.16 ± 0.16 | 5.31 ± 0.08 | 4.80 ± 0.14 | 4.06 ± 0.03 |
| Muscle | 1.08 ± 0.06 | 0.53 ± 0.05 | 0.37 ± 0.04 | 0.17 ± 0.01 | 0.11 ± 0.01 | 0.08 ± 0.01 |
| Blood | 3.40 ± 0.14 | 1.18 ± 0.08 | 0.91 ± 0.01 | 0.53 ± 0.01 | 0.18 ± 0.00 | 0.12 ± 0.01 |
| All bone | 7.22 ± 0.33 | 11.3 ± 1.76 | 11.4 ± 0.05 | 12.6 ± 0.13 | 17.6 ± 0.42 | 11.4 ± 0.31 |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Qiu, L.; Cheng, W.; Lin, J.; Luo, S.; Xue, L.; Pan, J. Synthesis and Biological Evaluation of Novel 99mTc-Labelled Bisphosphonates as Superior Bone Imaging Agents. Molecules 2011, 16, 6165-6178. https://doi.org/10.3390/molecules16086165
Qiu L, Cheng W, Lin J, Luo S, Xue L, Pan J. Synthesis and Biological Evaluation of Novel 99mTc-Labelled Bisphosphonates as Superior Bone Imaging Agents. Molecules. 2011; 16(8):6165-6178. https://doi.org/10.3390/molecules16086165
Chicago/Turabian StyleQiu, Ling, Wen Cheng, Jianguo Lin, Shineng Luo, Li Xue, and Jing Pan. 2011. "Synthesis and Biological Evaluation of Novel 99mTc-Labelled Bisphosphonates as Superior Bone Imaging Agents" Molecules 16, no. 8: 6165-6178. https://doi.org/10.3390/molecules16086165




