Synthesis and Biological Activity of New 1,3-Dioxolanes as Potential Antibacterial and Antifungal Compounds
Abstract
:1. Introduction
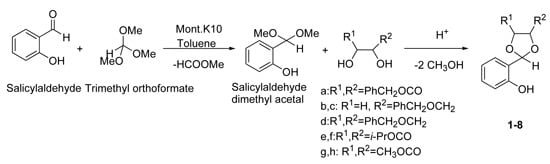
2. Results and Discussion
2.1. Chemistry

| Entry | Diol | Product | Reaction Time (h) | ee b (%) | Yield c (%) |
|---|---|---|---|---|---|
| 1 | a |  | 4 | >99 | 45 |
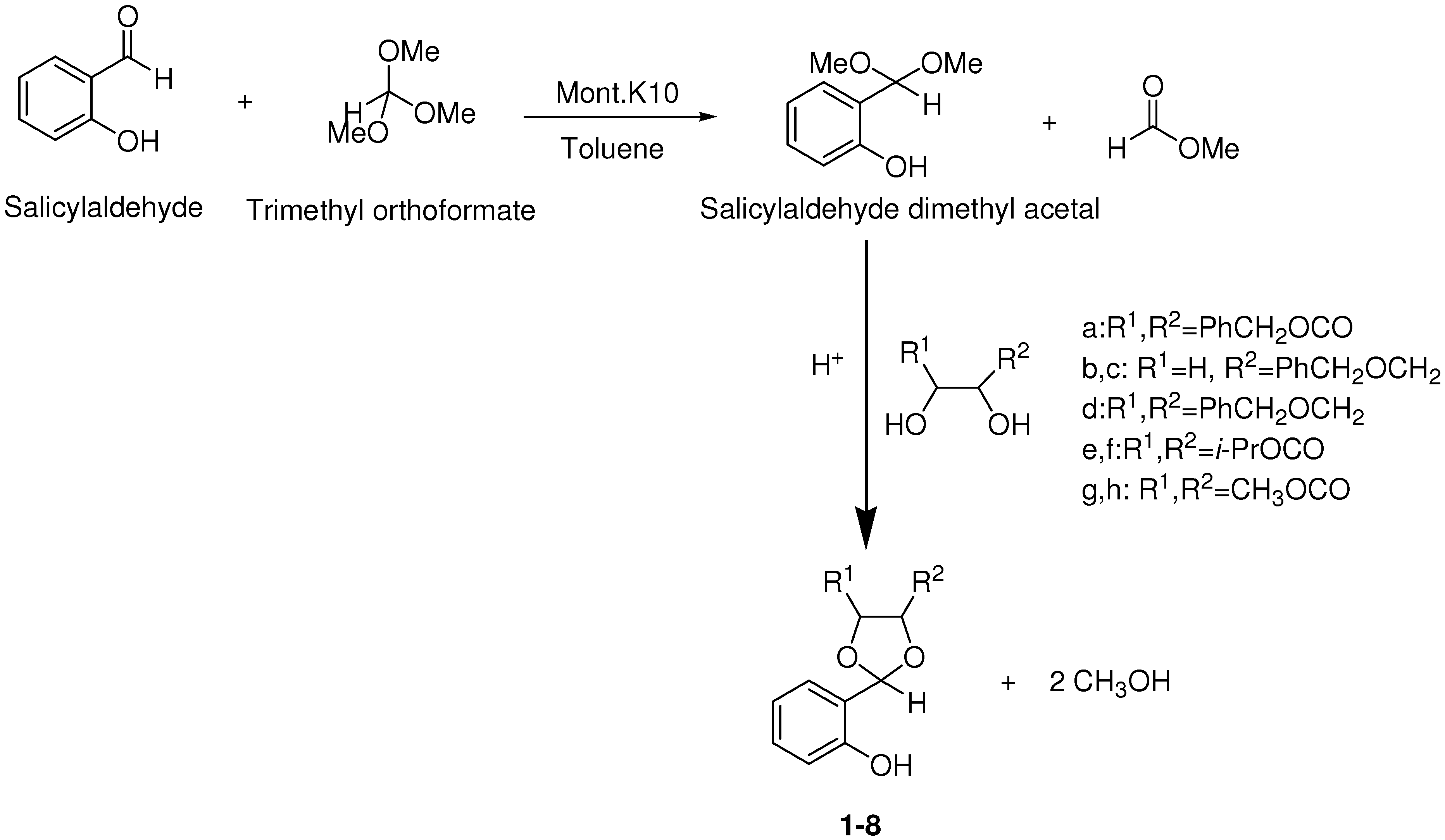
| 2 | b | 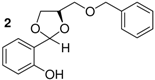 | 6 | >99 | 53 |
| 3 | c |  | 6 | − | 55 |
| 4 | d |  | 5 | >99 | 61 |
| 5 | e |  | 1 | >99 | 88 |
| 6 | f |  | 1 | − | 90 |
| 7 | g |  | 1 | >99 | 92 |
| 8 | h | 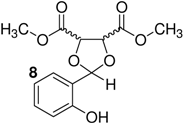 | 1 | − | 93 |
2.2. Biological Activity
| Microorganisms | MIC Values (µg/mL) | ||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||
| S. aureus | 1250 | 1250 | 1250 | 312.5 | 625 | 625 | - | 1250 | |
| S. epidermidis | 625 | 1250 | - | 625 | 1250 | 625 | 1250 | 625 | |
| E. faecalis | - | - | - | 625 | - | - | - | - | |
| P. aeruginosa | - | - | - | 625 | - | 625 | - | 625 | |
| E. coli | - | -. | - | - | - | - | - | - | |
| K. pneumoniae | - | - | - | - | - | - | - | - | |
| P. mirabilis | - | - | - | - | - | - | - | - | |
| C. albicans | - | 312.5 | 78.12 | 312.5 | 156.25 | 156.25 | 312.5 | 312.5 | |
3. Experimental
3.1. General
3.2. General Procedure for the Synthesis of 1,3-Dioxolanes 1-8
3.3. Biological Assays
4. Conclusions
Acknowledgments
Conflict of Interest
References and Notes
- Greene, T.W.; Wuts, P.G.M. Protective Groups in Organic Synthesis, 2nd ed; John Wiley & Sons: New York, NY, USA, 1991; pp. 129–133. [Google Scholar]
- Kociensky, P.J. Protecting Groups; Georg Thieme Verlag: New York, NY, USA, 1994. [Google Scholar]
- Gemal, A.L.; Luche, J.-L. Lanthanoids in organic synthesis. 4. Selective ketalization and reduction of carbonyl groups. J. Org. Chem. 1979, 44, 4187–4189. [Google Scholar] [CrossRef]
- Baji, H.; Kimny, T.; Gasquez, F.; Flammang, M.; Compagnon, P.L.; Delcourt, A.; Mathieu, G.; Viossat, B.; Morgant, G.; Nguyen-Huy, D. Synthesis, antifungal activity and structure-activity relationships of 2-(alkyl or aryl)-2-(alkyl or polyazol-1-ylmethyl)-4-(polyazol-1-ylmethyl)-1,3-dioxolanes. Eur. J. Med. Chem. 1997, 32, 637–650. [Google Scholar] [CrossRef]
- Crawley, G.C.; Briggs, M.T. Chiral dioxolane inhibitors of leukotriene biosynthesis: Structure-activity relationships and syntheses using asymmetric dihydroxylation. J. Med. Chem. 1995, 38, 3951–3956. [Google Scholar] [CrossRef]
- Genta, M.T.; Villa, C.; Mariani, E.; Loupy, A.; Petit, A.; Rizzetto, R.; Mascarotti, A.; Morini, F.; Ferro, M. Microwave-assisted preparation of cyclic ketals from a cineole ketone as potential cosmetic ingredients: solvent-free synthesis, odour evaluation, in vitro cytotoxicity and antimicrobial assays. Int. J. Pharm. 2002, 231, 11–20. [Google Scholar] [CrossRef]
- Shirai, R.; Takayama, H.; Nishikawa, A.; Koiso, Y.; Hashimoto, Y. Asymmetric synthesis of antimitotic combretadioxolane with potent antitumor activity against multi-drug resistant cells. Bioorg. Med. Chem. Lett. 1998, 8, 1997–2000. [Google Scholar] [CrossRef]
- Nguyen-Ba, N.; Lee, N.; Chan, L.; Zacharie, B. Synthesis and antiviral activities of N-9-oxypurine 1,3-Dioxolane and 1,3-oxathiolane nucleosides. Bioorg. Med. Chem. Lett. 2000, 10, 2223–2226. [Google Scholar] [CrossRef]
- Bera, S.; Malik, L.; Bhat, B.; Carrol, S.S.; MacCoss, M.; Olsen, D.B.; Tomassini, J.E.; Eldrup, A.B. Synthesis and evaluation of optically pure dioxolanes as inhibitors of hepatitis C virus RNA replication. Bioorg. Med. Chem. Lett. 2003, 13, 4455–4458. [Google Scholar] [CrossRef]
- Aepkers, M.; Wünsch, B. Structure–affinity relationship studies of non-competitive NMDA receptor antagonists derived from dexoxadrol and etoxadrol. Bioorg. Med. Chem. Lett. 2005, 13, 6836–6849. [Google Scholar] [CrossRef]
- Zapata-Sudo, G.; Pontes, L.B.; Gabriel, D.; Mendes, T.C.F.; Ribeiro, N.M.; Pinto, A.C.; Trachez, M.M.; Sudo, R.T. Sedative–hypnotic profile of novel isatin ketals. Pharmacol. Biochem. Behav. 2007, 86, 678–685. [Google Scholar] [CrossRef]
- Özkanlı, F.; Güney, A.; Calıs, U.; Uzbay, T. Synthesis and anticonvulsant activities of some new dioxolane derivatives. Arzneim.-Forsch. Drug Res. 2003, 53, 758–762. [Google Scholar]
- Pourjavadi, A.; Mirjalili, B.F. Microwave-assisted rapid ketalization/acetalization of aromatic aldehydes and ketones in aqueous media. J. Chem. Res (S) 1999, 562–563. [Google Scholar] [CrossRef]
- Li, T.S.; Li, S.H.; Li, J.T.; Li, H.Z. Montmorillonite clay catalysis. Part 2.1 An efficient and convenient procedure for the preparation of acetals catalysed by montmorillonite K-10. J. Chem. Res. (S) 1997, 26–27. [Google Scholar]
- Banik, B.K.; Chapa, M.; Marquez, J.; Cardona, M. A remarkable iodine-catalyzed protection of carbonyl compounds. Tetrahedron Lett. 2005, 46, 2341–2343. [Google Scholar] [CrossRef]
- Başpınar-Küçük, H.; Yusufoğlu, A. Synthesis of New Chiral and Racemic 1,3-Dioxolanes. J. Heterocycl. Chem. 2011. [Google Scholar] [CrossRef]
- Seebach, D.; K.Beck, A.; Heckel, A. TADDOLs, their derivatives, and TADDOL analogues: Versatile chiral auxiliaries. Angew. Chem. Int. Ed. 2001, 40, 92. [Google Scholar] [CrossRef]
- Furlan, R.L.E.; Mata, E.G.; Mascaretti, O.A. Butylstannonic acid catalyzed transesterification of carboxylic esters. Tetrahedron Lett. 1998, 39, 2257–2260. [Google Scholar]
- Iguchi, K.; Kitade, M.; Kashiwagi, T.; Yamada, Y. Structure and synthesis of petrosynes, new acetylenic enol ether glycerides from the Okinawan marine sponge of the genus Petrosia. J. Org. Chem. 1993, 58, 5690–5698. [Google Scholar] [CrossRef]
- Tuyet, T.M.T.; Harada, T.; Hashimoto, K.; Hatsuda, M.; Oku, A. Asymmetric synthesis of axially chiral biaryls via desymmetrization of 2,2’,6,6’-tetrahydroxybiphenyl using 1,4-Di-O-benzyl-L-threitol as a chiral template. J. Org. Chem. 2000, 65, 1335–1343. [Google Scholar]
- Fukuzawa, S.; Tsuchimoto, T.; Hotaka, T.; Hiyama, T. Direct synthesis of chiral acetals from carbonyl compounds and chiral diols: Sequential one-pot asymmetric silylcyanation reaction catalyzed by scandium(III) triflate. Synlett 1995, 1077–1078. [Google Scholar]
- Takabe, K.; Sugiura, M.; Asumi, Y.; Mase, N.; Yoda, H.; Shimizu, H. Practical optical resolution of dl-muscone using tartaric acid derivatives as a chiral auxiliary. Tetrahedron Lett. 2005, 46, 3457–3460. [Google Scholar]
- Tanaka, A.; Otsuka, S.; Yamashita, K. A practical synthesis of 1,2-O-Isopropylidene-(S)-glyceraldehyde. Agric. Biol. Chem. 1984, 48, 2135–2136. [Google Scholar] [CrossRef]
- Bruckner, R. Advanced Organic Chemistry Reaction Mechanisms; A Harcourt Science and Technology Company: San Diego, CA, USA, 2002; pp. 291–292. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI), Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 7th ed; CLSI: Wayne, PA, USA, 2006; Approved Standard M7-A7.
- Clinical and Laboratory Standards Institute (CLSI), Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standard–Second Edition; CLSI: Wayne, PA, USA, 1997; M27-A2.
- Clinical and Laboratory Standards Institute (CLSI), Performance Standards for Antimicrobial Susceptibility Testing: 7th Informational Supplement; CLSI: Wayne, PA, USA, 2010; M100-S20.
- Sample Availability: Samples of the compounds are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Küçük, H.B.; Yusufoğlu, A.; Mataracı, E.; Döşler, S. Synthesis and Biological Activity of New 1,3-Dioxolanes as Potential Antibacterial and Antifungal Compounds. Molecules 2011, 16, 6806-6815. https://doi.org/10.3390/molecules16086806
Küçük HB, Yusufoğlu A, Mataracı E, Döşler S. Synthesis and Biological Activity of New 1,3-Dioxolanes as Potential Antibacterial and Antifungal Compounds. Molecules. 2011; 16(8):6806-6815. https://doi.org/10.3390/molecules16086806
Chicago/Turabian StyleKüçük, Hatice Başpınar, Ayşe Yusufoğlu, Emel Mataracı, and Sibel Döşler. 2011. "Synthesis and Biological Activity of New 1,3-Dioxolanes as Potential Antibacterial and Antifungal Compounds" Molecules 16, no. 8: 6806-6815. https://doi.org/10.3390/molecules16086806