Synthesis and in Vitro Antimicrobial Activity of Some Pyrazolyl-1-carboxamide Derivatives
Abstract
:1. Introduction
2. Results and Discussion

2.1. Antimicrobial Activity
| Compound | X | Yield (%) | M.P.°C | Molecular Formula | Calculated % | Found % | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | H | N | C | H | N | |||||
| 2a | H | 76 | 161 | C13H15N3O | 68.12 | 6.55 | 18.34 | 68.06 | 6.49 | 18.31 |
| 2b | OCH3 | 82 | 171 | C14H17N3O2 | 64.86 | 6.56 | 16.22 | 64.85 | 6.48 | 16.19 |
| 2c | CH3 | 80 | 174 | C14H17N3O | 69.14 | 7.00 | 17.28 | 69.09 | 6,97 | 17.31 |
| 2d | Cl | 71 | 163 | C13H14N3ClO | 59.09 | 5.30 | 15.91 | 59.11 | 5.31 | 15.88 |
| 2e | Br | 89 | 179 | C13H14N3BrO | 50.65 | 4.55 | 13.64 | 50.69 | 4.52 | 13.51 |
| 2f | NO2 | 93 | 182 | C13H14N4O3 | 56.93 | 5.11 | 20.44 | 56.95 | 5.13 | 20.47 |
| 3a | H | 77 | 193 | C13H13N3O | 68.72 | 5.73 | 18.50 | 68.77 | 5.69 | 18.49 |
| 3b | OCH3 | 66 | 183 | C14H15N3O2 | 65.37 | 8.84 | 16.34 | 65.39 | 5.76 | 16.38 |
| 3c | CH3 | 67 | 176 | C14H15N3O | 69.71 | 6.22 | 17.43 | 69.75 | 6.19 | 17.44 |
| 3d | Cl | 81 | 169 | C13H12N3ClO | 59.54 | 4.58 | 16.03 | 59.60 | 4.58 | 16.04 |
| 3e | Br | 88 | 170 | C13H12N3BrO | 50.98 | 3.92 | 13.73 | 50.94 | 3.89 | 13.80 |
| 3f | NO2 | 91 | 199 | C13H12N4O3 | 57.35 | 4.41 | 20.59 | 57.32 | 4.43 | 20.51 |
| 4a | H | 69 | 188 | C15H15N3O2 | 66.91 | 5.58 | 15.61 | 66.88 | 5.56 | 15.66 |
| 4b | OCH3 | 62 | 172 | C16H17N3O3 | 64.21 | 5.69 | 14.05 | 64.19 | 5.66 | 14.08 |
| 4c | CH3 | 73 | 181 | C16H17N3O2 | 67.84 | 6.01 | 14.84 | 67.91 | 6.03 | 14.89 |
| 4d | Cl | 82 | 169 | C15H14N3ClO2 | 59.21 | 4.61 | 13.82 | 59.17 | 4.62 | 13.78 |
| 4e | Br | 71 | 176 | C15H14N3BrO2 | 51.72 | 4.02 | 12.07 | 51.73 | 4.07 | 12.03 |
| 4f | NO2 | 79 | 197 | C15H14N4O4 | 57.32 | 4.46 | 17.83 | 57.32 | 4.46 | 17.80 |
| 5a | H | 71 | 152 | C13H15N3O | 68.12 | 6.55 | 18.34 | 68.16 | 6.49 | 18.36 |
| 5b | OCH3 | 62 | 149 | C14H17N3O2 | 64.86 | 6.56 | 16.22 | 64.89 | 6.51 | 16.21 |
| 2c | CH3 | 69 | 143 | C14H17N3O | 69.14 | 7.00 | 17.28 | 69.17 | 6.97 | 17.29 |
| 5d | Cl | 77 | 161 | C13H14N3ClO | 59.09 | 5.30 | 15.91 | 59.11 | 5.27 | 15.88 |
| 5e | Br | 60 | 158 | C13H14N3BrO | 50.65 | 4.55 | 13.64 | 50.59 | 4.57 | 13.67 |
| 5f | NO2 | 77 | 169 | C13H14N4O3 | 56.93 | 5.11 | 20.44 | 56.97 | 5.17 | 20.39 |
| Comp. | IR cm−1 (KBr) | 1H-NMR (δ / ppm) a | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VinylHC =CH orAr– C=C | C=N | C=O | NHand/orNH2 | Ar-H’S and =C- CH =CH (m) | =C-CH=CH (d), J=12 Hz | Pyrazole C4–H (s) | Pyrazoline–HAdd,JAX = 3.6Hz, dd, JAB = 16Hz | Pyrazoline–HB dd,JAB = 16Hz, dd,JBX = 12Hz | Pyrazoline–HX dd,JAX = 3.6Hz, dd,JBX=12Hz | NH and/or NH2 (s), D2O exchangeable | Cyclopropyl ring H’S | Ar–CH3, Ar–OCH3, CH3CO–(S) | ||
| CH(m) | 2(CH2) (m) | |||||||||||||
| 2a | 1603 | 1631 | 1664 | 3234 and 3402 | 7.31–7.76 | 6.77 | – | – | – | – | 10.11, 10.63 | 1.89–2.54 | 0.73–1.36 | – |
| 2b | 1607 | 1633 | 1661 | 3235 and 3390 | 7.29–7.86 | 6.81 | – | – | – | – | 10.31, 10.57 | 1.83–2.36 | 0.72–1.38 | 3.66 |
| 2c | 1597 | 1627 | 1669 | 3240 and 3401 | 7.26–7.74 | 6.79 | – | – | – | – | 9.37, 10.42 | 1.84–2.42 | 0.69–1.41 | 2.22 |
| 2d | 1604 | 1645 | 1668 | 3227 and 3400 | 7.19–7.89 | 6.84 | – | – | – | – | 9.87, 10.73 | 1.79–2.41 | 0.75–1.36 | – |
| 2e | 1608 | 1650 | 1660 | 3222 and 3409 | 7.17–7.77 | 6.87 | – | – | – | – | 9.91, 10.61 | 1.71–2.45 | 0.78–1.26 | – |
| 2f | 1598 | 1663 | 1671 | 3228 and 3411 | 7.12–7.81 | 6.93 | – | – | – | – | 9.77, 10.82 | 1.63–2.67 | 0.77–1.31 | – |
| 3a | 1597 | 1634 | 1652 | 3387 | 7.24–7.86 b | – | 6.83 | – | – | – | 10.54 | 1.76–2.53 | 0.74–1.34 | – |
| 3b | 1593 | 1629 | 1660 | 3381 | 7.26–8.02 b | – | 6.74 | – | – | – | 10.61 | 1.81–2.33 | 0.71–1.36 | 3.71 |
| 3c | 1587 | 1633 | 1665 | 3401 | 7.21–7.98 b | – | 6.82 | – | – | – | 10.33 | 1.87–2.39 | 0.67–1.39 | 2.29 |
| 3d | 1591 | 1644 | 1659 | 3397 | 7.13–7.79 b | – | 6.81 | – | – | – | 10.39 | 1.66–2.43 | 0.69–1.32 | – |
| 3e | 1617 | 1650 | 1655 | 3395 | 7.11–7.75 b | – | 6.73 | – | – | – | 10.31 | 1.72–2.49 | 0.66–1.33 | – |
| 3f | 1614 | 1657 | 1670 | 3402 | 7.31–7.64 b | – | 6.79 | – | – | – | 10.70 | 1.70–2.62 | 0.71–1.29 | – |
| 4a | 1607 | 1634 | 1659 | 3230 | 7.25–7.83 b | – | 6.84 | – | – | – | 9.39 | 1.77–2.54 | 0.72–1.31 | 2.11 |
| 4b | 1602 | 1636 | 1660 | 3261 | 7.23–7.91 b | – | 6.75 | – | – | – | 9.29 | 1.79–2.55 | 0.73–1.37 | 2.13,3.69 |
| 4c | 1601 | 1622 | 1663 | 3233 | 7.23–7.89 b | – | 6.81 | – | – | – | 9.30 | 1.82–2.41 | 0.70–1.36 | 2.13,2.21 |
| 4d | 1598 | 1639 | 1662 | 3241 | 7.17–7.83 b | – | 6.86 | – | – | – | 9.27 | 1.69–2.43 | 0.72–1.32 | 2.14 |
| 4e | 1603 | 1651 | 1663 | 3237 | 7.16–7.73 b | – | 6.77 | – | – | – | 9.23 | 1.71–2.53 | 0.69–2.39 | 2.17 |
| 4f | 1611 | 1657 | 1676 | 3227 | 7.33–7.60 b | – | 6.87 | – | – | – | 9.37 | 1.73–2.59 | 0.73–2.58 | 2.18 |
| 5a | – | 1635 | 1657 | 3398 | 7.21–7.79 b | – | – | 3.09 | 3.79 | 5.42 | 10.63 | 1.74–2.51 | 0.71–1.32 | – |
| 5b | – | 1638 | 1659 | 3387 | 7.26–7.89 b | – | – | 3.11 | 3.71 | 5.45 | 10.65 | 1.76–2.53 | 0.76–1.36 | 3.67 |
| 5c | – | 1627 | 1661 | 3403 | 7.22–7.87 b | – | – | 3.07 | 3.72 | 5.39 | 10.57 | 1.80–2.41 | 0.69–1.40 | 2.24 |
| 5d | – | 1634 | 1667 | 3396 | 7.19–7.74 b | – | – | 3.13 | 3.81 | 5.44 | 10.49 | 1.72–2.47 | 0.76–1.45 | – |
| 5e | – | 1660 | 1668 | 3391 | 7.15–7.77 b | – | – | 3.14 | 3.77 | 5.37 | 10.44 | 1.70–2.55 | 0.69–2.43 | – |
| 5f | – | 1667 | 1679 | 3398 | 7.36–6.69 | – | – | 3.17 | 3.86 | 5.40 | 10.56 | 1.75–2.61 | 0.78–2.60 | – |
| Compound | X | Sta staphylococcus | Escherichia coli | Candida albicans |
|---|---|---|---|---|
| 3a | H | ++ | + | + |
| 3b | OCH3 | ++ | ++ | ++ |
| 3c | CH3 | ++ | ++ | + + |
| 3d | Cl | +++ | +++ | +++ |
| 3e | Br | ++ | + | ++ |
| 3f | NO2 | +++ | +++ | +++ |
| 4a | H | + | − | ++ |
| 4b | OCH3 | + | + | + |
| 4c | CH3 | − | − | + |
| 4d | Cl | ++ | ++ | ++ |
| 4e | Br | ++ | ++ | ++ |
| 4f | NO2 | +++ | +++ | +++ |
| 5a | H | + | − | + |
| 5b | OCH3 | + | − | ++ |
| 5c | CH3 | + | − | + |
| 5d | Cl | ++ | ++ | ++ |
| 5e | Br | ++ | ++ | ++ |
| 5f | NO2 | +++ | +++ | +++ |
3. Experimental
3.1. General
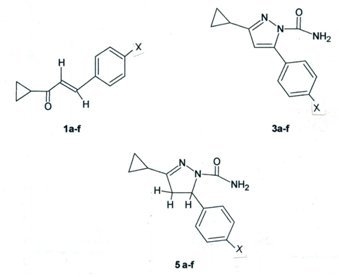
3.2. General Procedure for Preparation of E-1-Cyclopropyl-3-(p-substituted-phenyl)-2-propenones 1a-f
3.3. General Procedure for Preparation of 1-Cyclopropyl-3-(p-substituted-phenyl)-2-propene-1-semicarbazones 2a-f
3.4. General Procedure for Preparation of 3-Cyclopropyl-5-(p-substituted-phenyl)-pyrazole-1-carbox-amides 3a-f
3.5. General Procedure for Preparation of 3-Cyclopropyl-5-(p-substituted-phenyl)-pyrazole-1-(N-acetyl)-carboxamides 4a-f
3.6. General Procedure for Preparation of 4,5-Dihydro-3-cyclopropyl-5-(p-substituted-phenyl)-pyrazole-1-carboxamides 5a-f
3.7. Determination of Antimicrobial Activity
4. Conclusions
References
- Hamada, N.M.M.; Sharshira, E.M. Synthesis and antimicrobial evaluation of some heterocyclic chalcone derivatives. Molecules 2011, 16, 2304–2312. [Google Scholar] [CrossRef]
- Kalirajan, R.; Sivakumar, S.U.; Jubie, S.; Gowramma, B.; Suresh, B. Synthesis and biological evaluation of some heterocyclic derivatives of chalcones. Int. J. Chem. Tech. Res. 2009, 1, 27–34. [Google Scholar]
- Korgaokar, S.S.; Patil, P.H.; Shah, M.J.; Parekh, H.H. Studies on pyrazolines: Preparation and antimicrobial activity of 3-(3'(p-chlorophenyesulphonamido-phenyl)-5-aryl-1H-acetylpyrazolines. Ind. J. Pharm. Sci. 1996, 58, 222–225. [Google Scholar]
- Amir, M.; Kumar, H.; Khan, S.A. Synthesis and pharmacological evaluation of pyrazoline derivatives as new anti-inflammatory and analgesic agents. Bioorg. Med. Chem. Lett. 2008, 18, 918–922. [Google Scholar] [CrossRef]
- Ali, M.A.; Siddiqui, A.A.; Shaharyar, M. Synthesis, structural activity relationship and anti-tubercular activity of novel pyrazoline derivatives. Eur. J. Med. Chem. 2007, 42, 268–275. [Google Scholar] [CrossRef]
- Bilgin, A.A.; Palaska, E.; Sunal, R. Studies on the synthesis and antidepressant activity of some 1-thiocarbamoyl-3,5-diphenyl-2-pyrazolines. Arzneim. Forsch. Drug Res. 1993, 43, 1041–1044. [Google Scholar]
- Prasad, Y.R.; Rao, A.L.; Prasoona, L.; Murali, K.; Kumar, P.R. Synthesis and antidepressant activity of some 1,3,5-triphenyl-2-pyrazolines and 3-(2H-hydroxynaphthalen-1-yl)-1,5-diphneyl-2-pyrazolines. Bioorg. Med. Chem. Lett. 2005, 15, 5030–5034. [Google Scholar] [CrossRef]
- Palaska, E.; Aytemir, M.; Uzbay, I.T.; Erol, D. Synthesis and antidepressant activities of some 3,5-diphenyl-2-pyrazolines. Eur. J. Med. Chem. 2001, 36, 539–543. [Google Scholar] [CrossRef]
- Nassar, E. Synthesis, (in vitro) antitumer and antimicrobial activity of some pyrazoline, pyridine and pyrimidine derivatives linked to indoles moiety. J. Am. Sci. 2010, 6, 463–471. [Google Scholar]
- Azarifar, D.; Shaebanzadeh, M. Synthesis and characterization of new 3,5-dinaphthyl substituted 2-pyrazolines and study of their antimicrobial activity. Molecules 2002, 7, 885–895. [Google Scholar] [CrossRef]
- Palaska, E.; Erol, D.; Demirdamar, R. Synthesis and antidepressant activities of some 1,3,5-triphenyl-2-pyrazolines. Eur. J. Med. Chem. 1996, 31, 43–47. [Google Scholar] [CrossRef]
- Dmytro, H.; Borys, Z.; Olexandr, V.; Lucjusz, Z.; Andrzej, G.; Roman, L. Synthesis of novel thiazolone-based compound containing pyrazoline moiety and evaluation of their anticancer activity. Eur. J. Med. Chem. 2009, 44, 1396–1404. [Google Scholar] [CrossRef]
- Mui, M.S.; Siew, B.N.; Buss, A.D.; Crasta, S.C.; Kah, L.G.; Sue, K.L. Synthesis of N-1 acidic functionality affording analogues with enhanced antiviral activity against HIV. Bioorg. Med. Chem. Lett. 2002, 12, 679–699. [Google Scholar]
- Turan-zitouni, G.; Chevallet, P.; Kilic, F.S.; Erol, K. Synthesis of some thiazolyl-pyrazoline derivatives and preliminary investigation of their hypotensive activity. Eur. J. Med. Chem. 2000, 35, 635–641. [Google Scholar] [CrossRef]
- Parmar, S.S.; Pandey, B.R.; Dwivedi, C.; Harbison, R.D. Anticonvulsant activity and monoamine oxidase inhibitory properties of 1,3,5-trisubstituted pyrazolines. J. Pharm. Sci. 1974, 63, 1152–1155. [Google Scholar] [CrossRef]
- Siddiqui, A.A.; Rahman, M.A.; Shaharyar, M.; Mishra, R. Synthesis and anticonvulsant activity of some substituted 3,5-diphenyl-2-pyrazoline-1-carboxamide derivatives. Chem. Sci. J. 2010, 8, 1–10. [Google Scholar]
- Soni, N.; Pande, K.; Kalsi, R.; Gupta, T.K.; Parmar, S.S.; Barthwal, J.P. Inhibition of rat brain monoamine oxidase and succinic dehydrogenase by anticonvulsant pyrazolines. Res. Commun. Pathol. Pharmacol. 1987, 56, 129–132. [Google Scholar]
- Hamada, N.M.; Farrag, R.M.; Nasr, F.N. Synthesis and biological effects of pyrazoline derivatives against the cotton leafworm, spodoptera littoralis boisd. Egypt J. Agric. Res. 1998, 76, 1017–1026. [Google Scholar]
- Hamada, N.M. Synthesis and Spectral studies of some novel pyrazole derivatives from chalcones precursors. Heterocycl. Commun. 2009, 15, 327–334. [Google Scholar]
- Urichuk, L.J.; Allison, K.; Holt, A.; Greensaw, A.J.; Baker, J.B. Comparison of neurochemical effects of the monoamine oxidase inhibitors phenelzine, moclobemide and brofaroamine in the rat after short- and long-term adminstration. J. Affect. Disord. 2000, 58, 135–144. [Google Scholar] [CrossRef]
- Elba, M.E. Reductive debromination of erythro-2,3-dibromo-1-cyclopropyl-3-p-substituted phenyl)-1-propanones. J. Pharm. Sci. 1995, 9, 43–46. [Google Scholar]
- Matter, U.E.; Pascual, C.; Pretsch, E.; Pross, A.; Simon, W.; Sternhell, S. Estimation of chemical shifts of olefinic protons using additive increments-II. The compilation of additive increments for 43 functional groups. Teterahedron 1969, 25, 691–697. [Google Scholar] [CrossRef]
- Faidallah, H.M.; Sharshira, E.M.; Basaif, S.A.; A.-Ba-oum, A.E.-K. Synthesis and spectral characterization of novel 1,3,4-oxadiazole and 1,2,4-triazole derivatives: Synthesis for potential pharmacological activities. Phosphor. Sulfur Silicon Relat. Elem. 2002, 177, 67–79. [Google Scholar] [CrossRef]
- Yale, H.L.; Losse, K.; Martines, J.; Holsing, M.; Perry, F.M.; Bernstein, J. Chemotherapy of experimental tuberculosis. VIII. The synthesis of acid hydrazides, their derivatives and related compounds. J. Am. Chem. Soc. 1953, 75, 1933–1942. [Google Scholar] [CrossRef]
- Yang, J.-F.; Cao, H.; Liu, H.; Li, Q.B.; Ma, Y.-M. Synthesis and bioactivity of novel bis heterocyclic compounds containing pyrazole and oxadiazoline. J. Chin. Chim. Soc. 2011, 58, 1–7. [Google Scholar] [CrossRef]
- Elba, M.E.; Darwish, A.I.; Mohamed, A.A.; El Sadany, S.K. Reactions of (E)-4-(p-substitutedphenyl)-3-phenyl-3-buten-2-ones with hydrazine derivatives. Egypt J. Chem. 2000, 43, 483–500. [Google Scholar]
- Kanagarajan, V.; Ezhilarasi, M.R.; Gopalakrishnan, M. One-pot ultrasound irradiation promoted synthesis and spectral characterization of an array of novel 1,1'-(5,5'-(1,4-phenylene)bis(3-aryl-1-H-pyrazole-5,1(4H, 5H-diyl) diethanones, a bis acetylated pyrazoles. Spectrochim. Acta Part A 2011, 78, 635–639. [Google Scholar] [CrossRef]
- Mády, Z.V.; Ozohanics, O.; Csampai, A.; Kudar, V.; Frigyes, D.; Sohár, P. Ferrocenyl pyrazolines: Preparation, structure, redox properties and DFT study on regioselective ring closur. J. Organomet. Chem. 2009, 694, 4185–4195. [Google Scholar] [CrossRef]
- Schelz, Z.; Molnar, J.; Hohmann, J. Antimicrobial and antiplasmid activities of essential oils. Fitoterapia 2006, 77, 279–285. [Google Scholar] [CrossRef]
- Nadar, P.A.; Rajasekaran, K. Kinetics of reaction of semicarbazide with p-substituted methyl styryl ketones. Ind. J. Chem. 1979, 17B, 302–304. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sharshira, E.M.; Hamada, N.M.M. Synthesis and in Vitro Antimicrobial Activity of Some Pyrazolyl-1-carboxamide Derivatives. Molecules 2011, 16, 7736-7745. https://doi.org/10.3390/molecules16097736
Sharshira EM, Hamada NMM. Synthesis and in Vitro Antimicrobial Activity of Some Pyrazolyl-1-carboxamide Derivatives. Molecules. 2011; 16(9):7736-7745. https://doi.org/10.3390/molecules16097736
Chicago/Turabian StyleSharshira, Essam Mohamed, and Nagwa Mohamed Mahrous Hamada. 2011. "Synthesis and in Vitro Antimicrobial Activity of Some Pyrazolyl-1-carboxamide Derivatives" Molecules 16, no. 9: 7736-7745. https://doi.org/10.3390/molecules16097736