Two New Triterpenoids from Lysimachia heterogenea Klatt and Evaluation of Their Cytotoxicity
Abstract
:1. Introduction
2. Results and Discussion
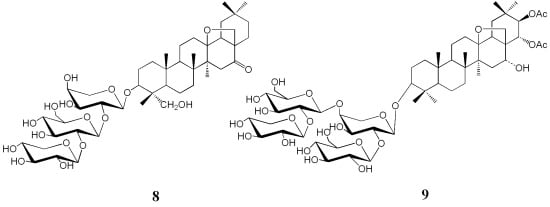
2.1. Structure Elucidation of New Compounds
| No. | Compound 8 | Compound 9 | ||
|---|---|---|---|---|
| C | H | C | H | |
| arabinose' | ||||
| 1 | 106.5 | 4.87 (d, 7.5 Hz) | 104.6 | 4.78 (d, 6.0 Hz) |
| 2 | 81.1 | 4.08 (brs) | 79.7 | 4.57 a |
| 3 | 73.9 | 4.25 a | 73.2 | 4.30 a |
| 4 | 74.5 | 4.31 a | 78.4 | 4.31 a |
| 5 | 66.5 | 3.65 (d, 12.5 Hz), 4.60 (d, 12.5 Hz) | 64.1 | 3.68 (m), 4.65 (dd, 12.0 Hz, 4 Hz) |
| glucose'' (at C-2 of arabinose) | ||||
| 1 | 105.3 | 5.01 (d, 7.5 Hz) | 104.9 | 5.50 (d, 7.5 Hz) |
| 2 | 86.2 | 3.95 a | 76.0 | 3.70-4.20 a |
| 3 | 77.9 | 3.80-4.30 a | 77.8 | 3.80-4.30 a |
| 4 | 71.0 | 4.25 a | 71.9 | 4.25 a |
| 5 | 78.2 | 3.80-4.30 a | 78.2 | 3.80-4.30 a |
| 6 | 62.4 | 4.32 (m), 4.45 (m) | 63.0 | 4.60 (m) |
| glucose''' (at C-4 of arabinose) | ||||
| 1 | 104.1 | 5.02 (d, 7.5 Hz) | ||
| 2 | 85.4 | 3.91 (m) | ||
| 3 | 77.6 | 3.80-4.30 a | ||
| 4 | 71.1 | 4.21 a | ||
| 5 | 77.9 | 3.80-4.30 a | ||
| 6 | 62.4 | 4.32 a | ||
| xylose (xylose''' for 8 and xylose'''' for 9) | ||||
| 1 | 108.0 | 4.89 (d, 6.5 Hz) | 107.6 | 4.93 (d, 6.0 Hz) |
| 2 | 76.2 | 3.80-4.30 a | 76.2 | 3.70-4.20 a |
| 3 | 77.6 | 3.80-4.30 a | 78.3 | 3.80-4.30 a |
| 4 | 70.4 | 3.80-4.30 a | 70.7 | 4.15 a |
| 5 | 67.2 | 3.48 (m), 4.28 a | 67.4 | 3.72 (m), 4.56 a |
| No. | Compound 8 | Compound 9 | ||
|---|---|---|---|---|
| C | H | C | H | |
| 1 | 39.1 | 1.02 a, 1.72 (m) | 39.2 | 0.85 a, 1.63 (m) |
| 2 | 26.1 | 2.02 (m), 2.23(m) | 26.5 | 1.90 (m) |
| 3 | 81.9 | 4.24 a | 88.9 | 3.12 (dd, 11.5 Hz, 4.0 Hz) |
| 4 | 43.6 | 39.7 | ||
| 5 | 47.4 | 1.58(m) | 55.6 | 0.63 (d, 11.5 Hz) |
| 6 | 17.5 | 1.66 (m) | 17.8 | 1.40 a |
| 7 | 33.6 | 1.04 a, 1.47 a | 34.2 | 1.32 a |
| 8 | 43.0 | 42.6 | ||
| 9 | 50.3 | 1.28 (m) | 50.4 | 1.23 a |
| 10 | 36.7 | 36.8 | ||
| 11 | 18.9 | 1.26 a, 1.51 a | 19.1 | 1.26-1.51 a |
| 12 | 31.7 | 1.52 (m) | 32.6 | 1.52 (m) |
| 13 | 86.2 | 86.0 | ||
| 14 | 49.8 | 44.7 | ||
| 15 | 45.8 | 1.91 (d, 15 Hz), 2.82 (d, 15 Hz) | 32.8 | 1.45 (m) |
| 16 | 212.0 | 78.4 | 3.80-4.30 a | |
| 17 | 56.1 | 50.9 | ||
| 18 | 54.6 | 2.01 (m) | 49.4 | 1.85 (m) |
| 19 | 40.0 | 1.40 (m) | 38.3 | 1.46 a, 2.64 (t, 15.0 Hz) |
| 20 | 31.8 | 37.1 | ||
| 21 | 35.6 | 1.19 (m), 1.79 (m) | 80.4 | 5.80 (d, 10.0 Hz) |
| 22 | 25.0 | 2.24 a | 74.3 | 4.30 a |
| 23 | 64.3 | 3.69 a, 4.33 a | 28.0 | 1.23 a |
| 24 | 13.3 | 0.96 (s) | 16.6 | 1.08 (s) |
| 25 | 16.7 | 0.94 (s) | 16.3 | 0.81 (s) |
| 26 | 18.8 | 1.32 (s) | 18.3 | 1.25 a |
| 27 | 21.7 | 1.01 (s) | 19.8 | 1.27 a |
| 28 | 75.1 | 3.50 (m) | 76.2 | 4.10 a |
| 29 | 33.3 | 0.86 (s) | 30.3 | 1.13 (s) |
| 30 | 23.5 | 0.81 (s) | 20.2 | 1.10 (s) |
| AcO (at C-21 of aglycon) | ||||
| CO | 171.1 | |||
| Me | 21.0 | 2.00 | ||
| AcO (at C-22 of aglycon) | ||||
| CO | 169.7 | |||
| Me | 21.9 | 2.40 | ||

3.2. The Cytotoxic Activity of Anagallisin C, Heterogenoside E and F
3. Experimental
3.1. General
3.2. Extraction and Isolation
3.3. Compound Characterization
3.4. Cytotoxicity Bioassays
4. Conclusions
Acknowledgments
Conflict of Interest
- Samples Availability: Samples of the compounds are available from the authors.
References
- Huang, X.A.; Liang, Y.J.; Cai, X.L.; Feng, X.Q.; Zhang, C.H.; Fu, L.W.; Deng, W.D. Four new cytotoxic oligosaccharidic derivatives of 12-oleanene from Lysimachia heterogenea Klatt. Bioorg. Med. Chem. Lett. 2009, 19, 6515–6518. [Google Scholar] [CrossRef]
- Huang, X.A.; Yang, R.Z. Progress in the triterpenoids from the genus Lysimachia L. J. Trop. Subtrop. Bot. 2007, 15, 175–182. [Google Scholar]
- Mahato, S.B.; Sahu, N.P.; Roy, S.K.; Sen, S. Structure elucidation of four new triterpenoid oligoglycosides from Anagallis arvensis. Tetrahedron 1991, 47, 5215–5230. [Google Scholar]
- Liu, H.W.; Zhang, X.; Gao, H.; Wang, N.L.; Jin, S.L.; Cai, B.; Yao, X.S.; Cai, G.P. Two new triterpenoid glycosides isolated from Aesculus assamica Griff. Chem. Pharm. Bull. 2005, 53, 1310–1313. [Google Scholar]
- Cao, S.G.; Norris, A.; Miller, J.S.; Ratovoson, F.; Razafitsalama, J.; Andriantsiferana, R.; Rasamison, V.E.; Kingston, D.G.I. Cytotoxic triterpenoid saponins of Albizia gummifera from the Madagascar rain forest. J. Nat. Prod. 2007, 70, 361–366. [Google Scholar]
- Huang, X.A.; Yang, R.Z. A new hydroquinone diglucoside from Lysimachia fordiana. Chem. Nat. Compd. 2004, 40, 457–459. [Google Scholar] [CrossRef]
- Huang, X.A.; Cai, J.Z.; Hu, Y.J.; Zhang, Y.H. Phytochemical investigation on Lysimachia fortunei. Chin. J. Chin. Mater. Med. 2007, 32, 596–599. [Google Scholar]
- Salah, H.B.; Jarraya, R.; Martin, M.T.; Veitch, N.C.; Grayer, R.J.; Simmonds, M.S.J.; Damak, M. Flavonol triglycosides from the leaves of Hammada scoparia (Pomel) Iljin. Chem. Pharm. Bull. 2002, 50, 1268–1270. [Google Scholar]
- Sukawa, K.; Sekine, H.; Takido, M. Two flavonol glycosides from Lysimachia fortunei. Phytochemistry 1989, 28, 2215–2216. [Google Scholar]
- Wei, Y.; Xie, Q.Q.; Ito, Y. Preparative separation of axifolin-3-glucoside, hyperoside and amygdalin from plant extracts by high-speed countercurrent chromatography. J. Liq. Chromatogr. Relat. Technol. 2009, 32, 1010–1022. [Google Scholar] [CrossRef]
- Huang, X.A.; Yang, R.Z.; Deng, W.D. A new poly-substituted benzaldehyde from the leaves of Lysimachia fordiana Oliv. Molecules 2007, 12, 43–48. [Google Scholar] [CrossRef]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Huang, X.-A.; Shen, X.-L.; Hu, Y.-J.; Liu, Y.-M.; Liu, K.-L.; Zhang, F.-X.; Zhou, X.-X. Two New Triterpenoids from Lysimachia heterogenea Klatt and Evaluation of Their Cytotoxicity. Molecules 2011, 16, 8076-8082. https://doi.org/10.3390/molecules16098076
Huang X-A, Shen X-L, Hu Y-J, Liu Y-M, Liu K-L, Zhang F-X, Zhou X-X. Two New Triterpenoids from Lysimachia heterogenea Klatt and Evaluation of Their Cytotoxicity. Molecules. 2011; 16(9):8076-8082. https://doi.org/10.3390/molecules16098076
Chicago/Turabian StyleHuang, Xin-An, Xiao-Ling Shen, Ying-Jie Hu, Ya-Ming Liu, Kang-Lun Liu, Feng-Xue Zhang, and Xin-Xin Zhou. 2011. "Two New Triterpenoids from Lysimachia heterogenea Klatt and Evaluation of Their Cytotoxicity" Molecules 16, no. 9: 8076-8082. https://doi.org/10.3390/molecules16098076