Synthesis of 1,4-Disubstituted Mono and Bis-triazolocarbo-acyclonucleoside Analogues of 9-(4-Hydroxybutyl)guanine by Cu(I)-Catalyzed Click Azide-Alkyne Cycloaddition
Abstract
:1. Introduction

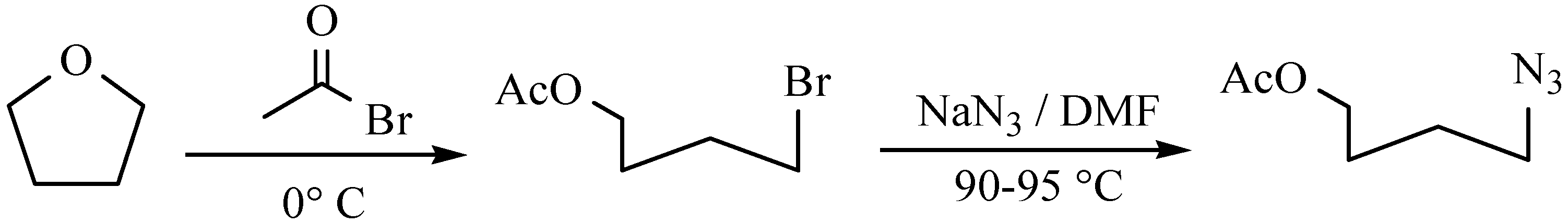
2. Results and Discussion
2.1. Chemistry

| Entry | Azide | Alkyne | Reaction time | Product a | Yields(% b) |
|---|---|---|---|---|---|
| 10a | 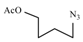 | 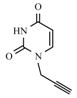 | 1 min | 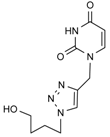 | 91 |
| 10b |  | 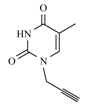 | 1 min | 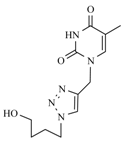 | 92 |
| 10c |  | 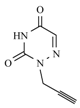 | 1 min | 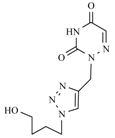 | 90 |
| 10d |  |  | 1 min | 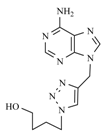 | 89 |
| 12a |  |  | 1 min | 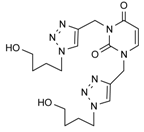 | 90 |
| 12b |  | 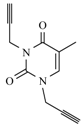 | 1 min | 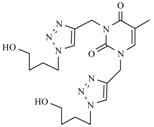 | 92 |
| 12c |  | 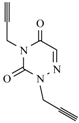 | 1 min | 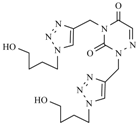 | 91 |

2.2. Biological Results
2.2.1. Antibacterial Activity
| Strains | Phenotype | Cipro | Lin | 10a | 10b | 10c | 10d | 12a | 12b | 12c | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | S. aureus | Sa1 | ATCC13709 in vivo | 0.12 | 1 | >64 | >64 | >64 | >64 | >64 | >64 | >64 |
| 2 | Sa26 | ATCC25923 | 0.25 | 1 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | |
| 3 | Sa26 + 10% Human serum | Serum effect | 0.25 | 1 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | |
| 4 | Sa26 + 50% Human serum | Serum effect | 0.5 | 2 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | |
| 5 | Sa4 | Oxford | 0.12 | 1 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | |
| 6 | Sa2 | MRSA, in vivo | 8 | 1 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | |
| 7 | E. faecalis | Ecalis1 | ATCC29212 VanS | 0,5 | 2 | >64 | >64 | >64 | >64 | >64 | >64 | >64 |
| 8 | E. faecium | Ecium1 | VanA | 16 | 0.5 | >64 | >64 | >64 | >64 | >64 | >64 | >64 |
| 9 | S. pneumoniae | Pn1 | ATCC49619 | 1 | 1 | >64 | >64 | >64 | >64 | >64 | >64 | >64 |
| 10 | Pn9 | PenR | 0.5 | 0.5 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | |
| 11 | Pn9+2.5% blood | Blood effect | 0.5 | 0.25 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | |
| 12 | H. influenzae | Hi3 | ATCC 31517 MMSA | ≤0.03 | 16 | >64 | >64 | >64 | >64 | >64 | >64 | >64 |
| 14 | E. coli | Ec1 | ATCC25922 | ≤0.03 | >32 | >64 | >64 | >64 | >64 | >64 | >64 | >64 |
| 16 | P. aeruginosa | Pa1 | ATCC 27853 | 0,25 | >32 | >64 | >64 | >64 | >64 | >64 | >64 | >64 |
3. Experimental
3.1. General
3.2. General Procedure for the Synthesis of Monopropargyl Heterocyclic Bases
3.3. General Procedure for the Synthesis of the N-1, N-3-Bis-propargylpyrimidines/as-Triazines
3.4. General Procedure for the Synthesis of the Triazole acyclonucleoside Derivatives
4. Conclusions
Acknowledgements
References and Notes
- Schinazi, R.F.; Mead, J.R.; Feorino, P.M. Insights into HIV chemotherapy. AIDS Res. Hum. Retrovirus. 1992, 8, 963–990. [Google Scholar] [CrossRef]
- Clercq, E.D. HIV inhibitors targeted at the reverse transcriptase. AIDS Res. Hum. Retrovirus. 1992, 8, 119–134. [Google Scholar] [CrossRef]
- EASL International Consensus Conference on Hepatitis C. Consensus Statement. J. Hepatol. 1999, 31, 3–8. [CrossRef]
- Wengel, J.; Schinazi, R.F.; Caruthers, M.H. Stereoselective synthesis, chemistry and antiviral evaluation of 1-(2,3-dideoxy-3-C-hydroxymethyl-beta-D-threo-pentofuranosyl)thymine derivatives. Bioorg. Med. Chem. 1995, 3, 1223–1229. [Google Scholar] [CrossRef]
- Sekiyama, T.; Hatsuya, S.; Tanaka, Y.; Uduyama, M.; Ono, N.; Iwayama, S.; Oikawa, M.; Suzuki, K.; Okunishi, M.; Tsuji, T. Synthesis and antiviral activity of novel acyclic nucleosides: Discovery of a cyclopropyl nucleoside with potent inhibitory activity against herpesviruses. J. Med. Chem. 1998, 41, 1248–1298. [Google Scholar]
- Nasr, M.; Litterst, C.; McGowan, J. Computer-assisted structure-activity correlations of dideoxynucleoside analogs as potential anti-HIV drugs. Antiviral Res. 1990, 14, 125–148. [Google Scholar] [CrossRef]
- Prasad, A.K.; Trikha, S.; Parmar, V.S. Nucleoside synthesis mediated by glycosyl transferring enzymes. Bioorg. Chem. 1999, 27, 135–154. [Google Scholar] [CrossRef]
- Chu, C.K.; Cutler, S.J.; Chu, C.K.; Cutler, S.J. Chemistry and antiviral activities of acyclonucleosides. J. Heterocycl. Chem. 1986, 23, 289–319. [Google Scholar]
- Parkanyi, C.; Yuan, H.L. Synthesis of acyclic nucleoside analogs of 6-substituted 2-aminopurines and 2-amino-8-azapurines. J. Heterocycl. Chem. 1990, 27, 1409–1413. [Google Scholar] [CrossRef]
- Pan, B.-C.; Chem, Z.-H.; Piras, G.; Dutschman, G.E.; Rowe, E.C.; Cheng, Y.-C.; Chu, S.-H. Synthesis and anti-HIV-1 activities of 6-arylthio and 6-arylselenoacyclonucleosides. J. Heterocycl. Chem. 1994, 31, 177–185. [Google Scholar] [CrossRef]
- Chen, W.; Flavin, M.T.; Filler, R.; Xu, Z.-Q. Synthesis and antiviral activities of fluorinated acyclic nucleoside phosphonates. J. Chem. Soc. Perkin Trans. 1 1998, 3979–3988. [Google Scholar]
- Hirota, K.; Monguchi, Y.; Sajiki, H.; Sako, M.; Kitada, Y. Novel synthesis of purine acyclonucleosides possessing a chiral 9-hydroxyalkyl group by sugar modification of 9-D-ribitylpurines. J.Chem. Soc. Perkin Trans. 1 1998, 941–946. [Google Scholar]
- Chu, C.K.; Baker, D.C. Nucleosides and Nucleotides as Antitumor and Antiviral Agents; Plenum Press: New York, NY, USA, 1993; pp. 101–113. [Google Scholar]
- El Ashry, E.S.H.; El Kilany, Y. Acyclonucleosides: Part 3. tri-, tetra-, and pentaseco-nucleosides. Adv. Heterocycl. Chem. 1998, 69, 129–215. [Google Scholar]
- El Ashry, E.S.H.; El Kilany, Y. Acyclonucleosides: Part 2. Diseco-nucleosides. Adv. Heterocycl. Chem. 1997, 68, 1–88. [Google Scholar] [CrossRef]
- El Ashry, E.S.H.; El Kilany, Y. Acyclonucleosides: Part 1. Seco-nucleosides. Adv. Heterocycl. Chem. 1996, 67, 391–438. [Google Scholar] [CrossRef]
- Rashed, N.; Abdel Hamid, H.; Ramadan, E.S.; El Ashry, E.S.H. Acyclo C-nucleoside analogues. Regioselective anellation of a triazole ring to 5-methyl-1,2,4-triazino[5,6-b]indole and formation of certain 3-poly hydroxyalkyl derivatives. Nucleos. Nucleot. 1998, 17, 1373–1384. [Google Scholar] [CrossRef]
- El Ashry, E.S.H.; El Kholy, I.E.; El Kilany, Y. Dehydrative ring-closure of 3-substituted 2-quinoxalinones to give fused and nonfused pyrazoloquinoxalines. Carbohydr. Res 1978, 60, 303–314. [Google Scholar] [CrossRef]
- Sidwell, R.W.; Huffman, J.H.; Khare, G.P.; Allen, L.B.; Witkowski, J.T.; Robins, R.K. Broad-spectrum antiviral activity of Virazole: 1-beta-D-Ribofuranosyl-1,2,4-triazole-3-carboxamide. Science 1972, 177, 705–706. [Google Scholar]
- Manfredini, S.; Vicentini, C.B.; Manfrini, M.; Bianchi, N.; Rutigliano, A.; Mistiachi, C.; Gambari, R. Pyrazolo-triazoles as light activable dna cleaving agents. Bioorg. Med. Chem. 2000, 8, 2343–2346. [Google Scholar] [CrossRef]
- Gothelf, K.V.; Jørgensen, K.A. Asymmetric 1,3-dipolar cycloaddition reactions. Chem. Rev. 1998, 98, 863–910. [Google Scholar]
- Lazrek, H.B.; Taourirte, M.; Oulih, T.; Barascut, J.L.; Imbach, J.L.; Pannecouque, C.; Witrouw, M.; De Clercq, E. Synthesis and anti-Hiv activity of new modified 1,2,3-triazole acyclonucleosides. Nucleos. Nucleot. 2001, 20, 1949–1960. [Google Scholar] [CrossRef]
- Rostovtsev Green, V.V.L.G.; Fokin, V.V.; Sharpless, K.B. Stepwise huisgen cycloaddition process: Copper(I)-catalyzed regioselective ligation of azides and terminal alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Tornfe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on solid phase: [1,2,3]-Triazoles by regiospecific copper(I)-talyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef]
- Wang, Q.; Chan, T.R.; Hilgraf, R.; Fokin, V.V.; Sharpless, K.B.; Finn, M.G. Bioconjugation by copper(I)-catalyzed azide-alkyne [3+2] cycloaddition. J. Am. Chem. Soc. 2003, 125, 3192–3193. [Google Scholar]
- Lober, S.; Rodriguez-Loaiza, P.; Gmeiner, P. Click linker: Efficient and high-yielding synthesis of a new family of SPOS resins by 1,3-dipolar cycloaddition. Org. Lett. 2003, 5, 1753–1755. [Google Scholar] [CrossRef]
- Pérez-Balderas, F.; Ortega-Munoz, M.; Morales-Sanfrutos, J.; Hernández-Mateo, F.; CalvoFlores, F.G.; Calvo-Asín, J.A.; Isac-García, J.; Santoyo González, F. Multivalent neoglycoconjugates by regiospecific cycloaddition of alkynes and azides using organic-soluble copper catalysts. Org. Lett. 2003, 5, 1951–1954. [Google Scholar]
- Krasinski, A.; Fokin, V.V.; Sharpless, K.B. Direct synthesis of 1,5-disubstituted-4-magnesio-1,2,3-triazoles, revisited. Org. Lett. 2004, 6, 1237–1240. [Google Scholar] [CrossRef]
- Kamijo, S.; Jin, T.; Huo, Z.; Yamamoto, Y. Synthesis of triazoles from nonactivated terminal alkynes via the three-component coupling reaction using a Pd(0)-Cu(I) bimetallic catalyst. J. Am. Chem. Soc. 2003, 125, 7786–7787. [Google Scholar]
- Kamijo, S.; Jin, T.; Huo, Z.; Yamamoto, Y. A one-pot procedure for the regiocontrolled synthesis of allyltriazoles via the Pd-Cu bimetallic catalyzed three-component coupling reaction of nonactivated terminal alkynes, allyl carbonate, and trimethylsilyl azide. J. Org. Chem. 2004, 69, 2386–2393. [Google Scholar]
- Yan, Z.Y.; Zhao, Y.B.; Fan, M.J.; Liu, W.M.; M.Liang, Y. General synthesis of (1-substituted-1H-1,2,3-triazol-4-ylmethyl)-dialkylamines via a copper(I)-catalyzed three-component reaction in water. Tetrahedron 2005, 61, 9331–9337. [Google Scholar]
- Chittepu, P.; Sirivolu, V.R.; Seela, F. Nucleosides and oligonucleotides containing 1,2,3-triazole residues with nucleobase tethers: Synthesis via the azide-alkyne ‘click’ reaction. Bioorg. Med. Chem. 2008, 16, 8427–8439. [Google Scholar]
- El Akri, K.; Bougrin, K.; Balzarini, B.J.; Faraj, A.; Benhida, R. Efficient synthesis and in vitro cytostatic activity of 4-substituted triazolyl-nucleosides. Bioorg. Med. Chem. Lett. 2007, 17, 6656–6659. [Google Scholar] [CrossRef]
- Krim, J.; Sillahi, B.; Taourirte, M.; Rakib, E.M.; Engels, J.W. Microwave-assisted click chemistry: Synthesis of mono and bis-1,2,3-triazole acyclonucleoside analogues of ACV via copper(I)-catalyzed cycloaddition. ARKIVOC 2009, xiii, 142–152. [Google Scholar]
- Lucas, R.; Neto, V.; Bouazza, A.H.; Zerrouki, R.; Granet, R.; Krausz, P.; Yves Champavier, Y. Microwave-assisted synthesis of a triazole-linked 3′-5′ dithymidine using click chemistry. Tetrahedron Lett. 2008, 49, 1004–1007. [Google Scholar] [CrossRef]
- Keller, P.M.; FyfeJ, A.; Beauchamp, L.; Lubbers, C.M.; Furman, P.A.; Schaeffer, H.J.; Elion, G.B. Enzymatic phosphorylation of acyclic nucleoside analogs and correlations with antiherpetic activities. Biochem. Pharmacol. 1981, 30, 3071–3077. [Google Scholar] [CrossRef]
- CLSI-Clinical and Laboratory Standards Institute, Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that grow Aerobically, Approved Standard M7-A7, 7th ed; CLSI: Wayne, PA, USA, 2006.
- Sample Availability: Not available.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Krim, J.; Taourirte, M.; Engels, J.W. Synthesis of 1,4-Disubstituted Mono and Bis-triazolocarbo-acyclonucleoside Analogues of 9-(4-Hydroxybutyl)guanine by Cu(I)-Catalyzed Click Azide-Alkyne Cycloaddition. Molecules 2012, 17, 179-190. https://doi.org/10.3390/molecules17010179
Krim J, Taourirte M, Engels JW. Synthesis of 1,4-Disubstituted Mono and Bis-triazolocarbo-acyclonucleoside Analogues of 9-(4-Hydroxybutyl)guanine by Cu(I)-Catalyzed Click Azide-Alkyne Cycloaddition. Molecules. 2012; 17(1):179-190. https://doi.org/10.3390/molecules17010179
Chicago/Turabian StyleKrim, Jamal, Moha Taourirte, and Joachim W. Engels. 2012. "Synthesis of 1,4-Disubstituted Mono and Bis-triazolocarbo-acyclonucleoside Analogues of 9-(4-Hydroxybutyl)guanine by Cu(I)-Catalyzed Click Azide-Alkyne Cycloaddition" Molecules 17, no. 1: 179-190. https://doi.org/10.3390/molecules17010179




