Neuroprotective Effects of Exogenous Activin A on Oxygen-Glucose Deprivation in PC12 Cells
Abstract
:1. Introduction
2. Results
2.1. Effects of ActA on Cell Proliferation
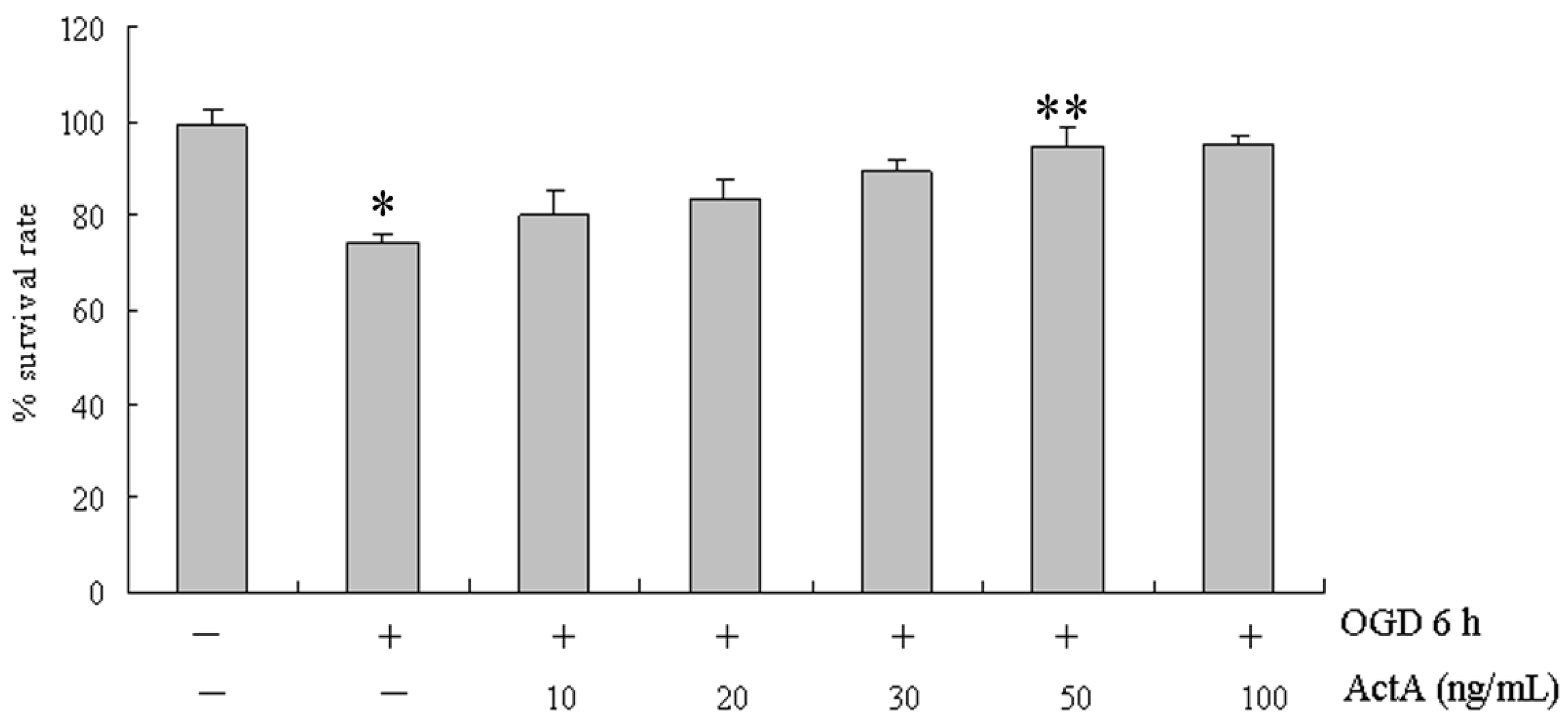
2.2. ActA Anti-Apoptotic in PC12 Cells
2.3. Caspase-3 Activation


2.4. Effects of ActA on ActA/Smad Pathway

2.5. Inhibition of NO and iNOS by ActA

2.6. Increasing the Activity of SOD by ActA

3. Discussion
4. Experimental
4.1. Cell Culture
4.2. OGD for in Vitro Ischemia
4.3. Cell Viability Assay
4.4. Flow Cytometry Using Annexin V/PI Staining
4.5. Western Blotting Analysis
4.6. Caspase-3 Activation Assays
4.7. Measurement of NO

4.8. Measurement of SOD

4.9. Statistics
5. Conclusions
Acknowledgements
- Sample Availability: Samples of all compounds are available from the authors.
References and Notes
- Paeme, S.; Moorhead, K.T.; Chase, J.G.; Lambermont, B.; Kolh, P.; D’Orio, V.; Pierard, L.; Moonen, M.; Lancellotti, P.; Dauby, P.C.; et al. Mathematical multi-scale model of the cardiovascular system including mitral valve dynamics. Application to ischemic mitral insufficiency. Biomed. Eng. Online 2011, 10, 86. [Google Scholar] [CrossRef]
- Barbarash, O.L.; Zykov, M.V.; Kashtalap, V.V.; Barbarash, L.S. Prevalence and clinical significance of multifocal atherosclerosis in patients with ischemic heart disease. Kardiologiia 2011, 51, 66–71. [Google Scholar]
- Meschia, J.F.; Nalls, M.; Matarin, M.; Brott, T.G.; Brown, R.D., Jr.; Hardy, J.; Kissela, B.; Rich, S.S.; Singleton, A.; Hernandez, D.; et al. Siblings with ischemic stroke study: Results of a genome-wide scan for stroke loci. Stroke 2011, 42, 2726–2732. [Google Scholar] [CrossRef]
- Foster, G.P.; Westerdahl, D.E.; Foster, L.A.; Hsu, J.V.; Anholm, J.D. Ischemic preconditioning of the lower extremity attenuates the normal hypoxic increase in pulmonary artery systolic pressure. Respir. Physiol. Neurobiol. 2011, 179, 248–253. [Google Scholar] [CrossRef]
- Ageta, H.; Tsuchida, K. Multifunctional roles of activins in the brain. Vitam. Horm. 2011, 85, 185–206. [Google Scholar] [CrossRef]
- Deli, A.; Kreidl, E.; Santifaller, S.; Trotter, B.; Seir, K.; Berger, W.; Schulte-Hermann, R.; Rodgarkia-Dara, C.; Grusch, M. Activins and activin antagonists in hepatocellular carcinoma. World J. Gastroenterol. 2008, 14, 1699–1709. [Google Scholar]
- Kang, H.Y.; Shyr, C.R. Activins and cell migration. Vitam. Horm. 2011, 85, 129–148. [Google Scholar] [CrossRef]
- Munz, B.; Tretter, Y.P.; Hertel, M.; Engelhardt, F.; Alzheimer, C.; Werner, S. The roles of activins in repair processes of the skin and the brain. Mol. Cell. Endocrinol. 2001, 180, 169–177. [Google Scholar] [CrossRef]
- Florio, P.; Gazzolo, D.; Luisi, S.; Petraglia, F. Activin A in brain injury. Adv. Clin. Chem. 2007, 43, 117–130. [Google Scholar] [CrossRef]
- Mukerji, S.S.; Rainey, R.N.; Rhodes, J.L.; Hall, A.K. Delayed activin A administration attenuates tissue death after transient focal cerebral ischemia and is associated with decreased stress-responsive kinase activation. J. Neurochem. 2009, 111, 1138–1148. [Google Scholar] [CrossRef]
- Liu, W.B.; Zhou, J.; Qu, Y.; Li, X.; Lu, C.T.; Xie, K.L.; Sun, X.L.; Fei, Z. Neuroprotective effect of osthole on MPP+-induced cytotoxicity in PC12 cells via inhibition of mitochondrial dysfunction and ROS production. Neurochem. Int. 2010, 57, 206–215. [Google Scholar] [CrossRef]
- Chen, T.; Liu, W.; Chao, X.; Qu, Y.; Zhang, L.; Luo, P.; Xie, K.; Huo, J.; Fei, Z. Neuroprotective effect of osthole against oxygen and glucose deprivation in rat cortical neurons: Involvement of mitogen-activated protein kinase pathway. Neuroscience 2011, 183, 203–211. [Google Scholar] [CrossRef]
- Winkler, E.A.; Bell, R.D.; Zlokovic, B.V. Lack of Smad or Notch leads to a fatal game of brain pericyte hopscotch. Dev. Cell 2011, 20, 279–280. [Google Scholar] [CrossRef]
- Phillips, D.J.; Nguyen, P.; Adamides, A.A.; Bye, N.; Rosenfeld, J.V.; Kossmann, T.; Vallance, S.; Murray, L.; Morganti-Kossmann, M.C. Activin a release into cerebrospinal fluid in a subset of patients with severe traumatic brain injury. J. Neurotraum. 2006, 23, 1283–1294. [Google Scholar] [CrossRef]
- Wu, D.D.; Lai, M.; Hughes, P.E.; Sirimanne, E.; Gluckman, P.D.; Williams, C.E. Expression of the activin axis and neuronal rescue effects of recombinant activin A following hypoxic-ischemic brain injury in the infant rat. Brain Res. 1999, 835, 369–378. [Google Scholar] [CrossRef]
- Tong, J.L.; Nie, F.; Ran, Z.H.; Pan, C.Q.; Xu, X.T.; Zhu, M.M.; Xiao, S.D. Epigallocatechin gallate induces apoptosis in human hepatocellular carcinoma HepG2 cells via TGF/Smad signaling pathway. Zhonghua Zhong Liu Za Zhi 2009, 31, 646–650. [Google Scholar]
- Yao, Q.; Pawlaczyk, K.; Ayala, E.R.; Styszynski, A.; Breborowicz, A.; Heimburger, O.; Qian, J.Q.; Stenvinkel, P.; Lindholm, B.; Axelsson, J. The role of the TGF/Smad signaling pathway in peritoneal fibrosis induced by peritoneal dialysis solutions. Nephron. Exp. Nephrol. 2008, 109, e71–e78. [Google Scholar] [CrossRef]
- Leonardi, A.; Di Stefano, A.; Motterle, L.; Zavan, B.; Abatangelo, G.; Brun, P. Transforming growth factor-beta/Smad—Signalling pathway and conjunctival remodelling in vernal keratoconjunctivitis. Clin. Exp. Allergy 2011, 41, 52–60. [Google Scholar] [CrossRef]
- Nakamura, M.; Ito, H.; Nakamura, Y.; Wate, R.; Kaneko, S.; Nakano, S.; Matsumoto, S.; Kusaka, H. Smad ubiquitination regulatory factor-2 in progressive supranuclear palsy. Neuropathol. Appl. Neurobiol. 2011, 37, 307–314. [Google Scholar] [CrossRef]
- Sachdev, U.; Cui, X.; Hong, G.; Namkoong, S.; Karlsson, J.M.; Baty, C.J.; Tzeng, E. High mobility group box 1 promotes endothelial cell angiogenic behavior in vitro and improves muscle perfusion in vivo in response to ischemic injury. J. Vasc. Surg. 2011, 55, 180–191. [Google Scholar]
- Templin, C.; Luscher, T.F.; Landmesser, U. Stem and progenitor cell-based therapy approaches: Current developments on treatment of acute myocardial infarction and chronic ischemic cardiomyopathy. Herz 2010, 35, 445–456. [Google Scholar] [CrossRef] [Green Version]
- De Meyer, S.F.; Schwarz, T.; Schatzberg, D.; Wagner, D.D. Platelet glycoprotein Ibalpha is an important mediator of ischemic stroke in mice. Exp. Transl. Stroke Med. 2011, 3, 9. [Google Scholar] [CrossRef]
- Shudo, Y.; Miyagawa, S.; Fukushima, S.; Saito, A.; Kawaguchi, N.; Matsuura, N.; Sawa, Y. Establishing new porcine ischemic cardiomyopathy model by transcatheter ischemia-reperfusion of the entire left coronary artery system for preclinical experimental studies. Transplantation 2011, 92, e34–e35. [Google Scholar] [CrossRef]
- Park, D.; Joo, S.S.; Lee, H.J.; Choi, K.C.; Kim, S.U.; Kim, Y.B. Microtubule-associated protein 2, an early blood marker of ischemic brain injury. J. Neurosci. Res. 2011, 90, 461–467. [Google Scholar]
- Li, C.T.; Zhang, W.P.; Lu, Y.B.; Fang, S.H.; Yuan, Y.M.; Qi, L.L.; Zhang, L.H.; Huang, X.J.; Zhang, L.; Chen, Z.; et al. Oxygen-glucose deprivation activates 5-lipoxygenase mediated by oxidative stress through the p38 mitogen-activated protein kinase pathway in PC12 cells. J. Neurosci. Res. 2009, 87, 991–1001. [Google Scholar] [CrossRef]
- Longxi, P.; Buwu, F.; Yuan, W.; Sinan, G. Expression of p53 in the effects of artesunate on induction of apoptosis and inhibition of proliferation in rat primary hepatic stellate cells. PLoS One 2011, 6, e26500. [Google Scholar]
- Rao, V.; Balachandran, B.; Shen, H.; Logan, A.; Rao, L. In vitro and in vivo antioxidant properties of the plant-based supplement greens+. Int. J. Mol. Sci. 2011, 12, 4896–4908. [Google Scholar] [CrossRef]
- Han, P.; Kang, J.H.; Li, H.L.; Hu, S.X.; Lian, H.H.; Qiu, P.P.; Zhang, J.; Li, W.G.; Chen, Q.X. Antiproliferation and apoptosis induced by tamoxifen in human bile duct carcinoma QBC939 cells via upregulated p53 expression. Biochem. Biophys. Res. Commun. 2009, 385, 251–256. [Google Scholar] [CrossRef]
- Tara, S.; Takagi, G.; Miyamoto, M.; Kirinoki-Ichikawa, S.; Yamamoto, T.; Takano, H.; Takagi, I.; Yasutake, M.; Tabata, Y.; Mizuno, K. Novel approach to ischemic skin ulcer in systemic lupus erythematosus: Therapeutic angiogenesis by controlled-release basic fibroblast growth factor. Geriatr. Gerontol. Int. 2011, 11, 527–530. [Google Scholar] [CrossRef]
- Pieri, A.; Lopes, T.O.; Gabbai, A.A. Stratification with CHA2DS2-VASc score is better than CHADS2 score in reducing ischemic stroke risk in patients with atrial fibrillation. Int. J. Stroke 2011, 6, 466. [Google Scholar]
- Peng, B.; Zhu, Y.; Cui, L.; Ni, J.; Xu, W.; Zhou, L.; Yao, M.; Chen, L.; Wang, J.; Wang, Y.; et al. Standard medical management in secondary prevention of ischemic stroke in China (SMART). Int. J. Stroke 2011, 6, 461–465. [Google Scholar] [CrossRef]
- Kim, J.S.; Yang, W.I.; Shim, C.Y.; Ha, J.W.; Chung, N.; Chang, H.J. Hemorrhagic transformation of ischemic stroke: Severe complications of prosthetic valve endocarditis. Korean Circ. J. 2011, 41, 490–493. [Google Scholar] [CrossRef]
- Kobusiak-Prokopowicz, M.; Jolda-Mydlowska, B.; Zubkiewicz, A.; Szymczak, M.; Mysiak, A.; Skalik, R. Impact of nebivolol on levels of serum nitric oxide, plasma von Willebrand factor and exercise stress testing parameters in hypertensive and ischemic heart disease patients. Cardiol. J. 2008, 15, 162–168. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
He, J.-T.; Mang, J.; Mei, C.-L.; Yang, L.; Wang, J.-Q.; Xing, Y.; Yang, H.; Xu, Z.-X. Neuroprotective Effects of Exogenous Activin A on Oxygen-Glucose Deprivation in PC12 Cells. Molecules 2012, 17, 315-327. https://doi.org/10.3390/molecules17010315
He J-T, Mang J, Mei C-L, Yang L, Wang J-Q, Xing Y, Yang H, Xu Z-X. Neuroprotective Effects of Exogenous Activin A on Oxygen-Glucose Deprivation in PC12 Cells. Molecules. 2012; 17(1):315-327. https://doi.org/10.3390/molecules17010315
Chicago/Turabian StyleHe, Jin-Ting, Jing Mang, Chun-Li Mei, Le Yang, Jiao-Qi Wang, Ying Xing, Hong Yang, and Zhong-Xin Xu. 2012. "Neuroprotective Effects of Exogenous Activin A on Oxygen-Glucose Deprivation in PC12 Cells" Molecules 17, no. 1: 315-327. https://doi.org/10.3390/molecules17010315



