The Effect of β-Carotene Supplementation on the Pharmacokinetics of Nelfinavir and Its Active Metabolite M8 in HIV-1-infected Patients
Abstract
:1. Introduction
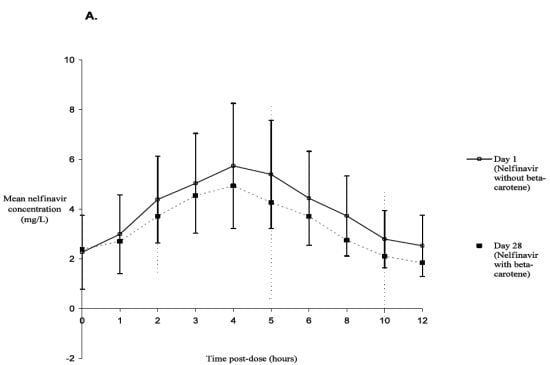
2. Results and Discussion
| Baseline characteristics | Day 28 characteristics (n = 11) | p † value | |
|---|---|---|---|
| (n = 11) | |||
| Age (years) | 45.5 ± 9.4 | - | - |
| Gender [n, (%) male] | 9 (81.8) | - | - |
| Weight (kg) | 76.6 ± 15.7 | 76.0 ± 16.6 | 0.489 |
| Body mass index (kg/m2) | 26.2 ± 4.0 | 26.1 ± 4.1 | 0.83 |
| Ethnicity [n, (%)] | |||
| - Caucasian | 6 (54.5) | - | - |
| - African / African-American | 4 (36.4) | - | - |
| - Asian | 1 (9.1) | - | - |
| CD4+ (cells/μL) | 616.2 ± 229.3 | 667.4 ± 330.5 | 0.258 |
| CD4+ % | 29.6 ± 8.5 | 33.3 ± 10.3 | 0.0009 |
| CD8+ (cells/μL) | 859.5 ± 435.1 | 759. 5 ± 413.0 | 0.091 |
| CD8+ % | 39.9 ± 13.2 | 37.8 ± 12.8 | 0.07 |
| CD4+:CD8+ ratio | 0.9 ± 0.6 | 1.1 ± 0.6 | 0.003 |
| Absolute lymphocyte count (×109/L) | 2.0 ± 0.7 | 2.0 ± 0.7 | 0.873 |
| n (%) with HIV viral load <50 copies/mL | 10 (90.9) | 10 (90.9) | - |
| (1 patient had 63) | (1 patient had 50) | ||
| Carotene level (μmol/L) | 3.35 ± 1.29 | 5.16 ± 1.61 | 0.0056 |
| (reference range: 1.0–5.5 μmol/L) |
| Pharmacokinetic parameter ∆ | Nelfinavir alone (day 1) | Nelfinavir and Beta-carotene (day 28) | GMR and 90% CI |
|---|---|---|---|
| Nelfinavir (n = 11) | |||
| AUC0–12 h (h*mg/L) | 35.46 (10.40–119.36) | 34.11 (11.37–69.61) | 0.90 (0.81–1.05) |
| Cmin (mg/L) | 1.35 (0.10–7.73) | 1.43 (0.20–2.41) | 1.04 (0.68–2.38) |
| Cmax (mg/L) | 5.20 (2.08–13.57) | 4.87 (2.03–10.34) | 0.96 (0.87–1.11) |
| Tmax (h) | 4.00 (2.00–5.00) | 4.00 (3.00–12.00) ¥ | |
| t½ (h) | 4.81 (2.65–14.40) | 4.24 (2.51–8.83) | |
| M8 (n = 9 § ) | |||
| AUC0–12 h (h*mg/L) | 10.18 (3.59–25.72) | 10.71 (4.91–22.05) | 0.99 (0.88–1.18) |
| Cmin (mg/L) | 0.30 (0.05–0.62) | 0.34 (0.09–0.54) | 1.31 (0.90–2.05) |
| Cmax (mg/L) | 1.53 (0.49–4.64) | 1.87 (0.95–3.56) | 1.02 (0.86–1.28) |
| Tmax (h) | 4.00 (3.00–5.00) | 4.00 (3.00–12.00) ¥ | |
| t½ (h) | 2.72 (1.90–5.77) | 3.21 (1.96–4.55) | |
| Metabolic AUC ratio (n = 9 § ) | |||
| AUC M8: AUC | |||
| nelfinavir | 0.33 (0.14–0.73) | 0.31 (0.17–0.72) | 1.00 (0.90–1.14) |


Discussion
3. Experimental
3.1. Study Design
3.2. Subjects
3.3. Study Drugs
3.4. Assessment and Data Abstraction
3.5. Pharmacokinetic Sample Collection
3.7. Pharmacokinetic Analysis
3.8. Statistical Analysis
3.9. Ethical Considerations
4. Conclusions
Acknowledgements
Conflict of Interest
- Sample Availability: Samples of nelfinavir [(3S,4aS,8aS)-N-tert-Butyl-2-[(2R,3R)-3-(3,2-cresotamido)-2-hydroxy-4-(phenylthio)butyl]decahydro-3-isoquinolinecarboxamide monomethanesulfonate] and M8 (Hydroxy-t-butylamidonelfinavir) may be available upon request from Pfizer Canada (Kirkland, Quebec, Canada).
References and Notes
- Singhal, N.; Austin, J. A clinical review of micronutrients in hiv infection. J. Int. Assoc. Physicians AIDS Care (Chic. Ill) 2002, 1, 63–75. [Google Scholar] [CrossRef]
- Fairfield, K.M.; Eisenberg, D.M.; Davis, R.B.; Libman, H.; Phillips, R.S. Patterns of use, expenditures, and perceived efficacy of complementary and alternative therapies in hiv-infected patients. Arch. Intern. Med. 1998, 158, 2257–2264. [Google Scholar] [CrossRef]
- Bogden, J.D.; Baker, H.; Frank, O.; Perez, G.; Kemp, F.; Bruening, K.; Louria, D. Micronutrient status and human immunodeficiency virus (hiv) infection. Ann. N. Y. Acad. Sci. 1990, 587, 189–195. [Google Scholar]
- Pace, G.W.; Leaf, C.D. The role of oxidative stress in hiv disease. Free Radic Biol. Med. 1995, 19, 523–528. [Google Scholar] [CrossRef]
- Keating, J.; Bjarnason, I.; Somasundaram, S.; Macpherson, A.; Francis, N.; Price, A.B.; Sharpstone, D.; Smithson, J.; Menzies, I.S.; Gazzard, B.G. Intestinal absorptive capacity, intestinal permeability and jejunal histology in hiv and their relation to diarrhoea. Gut 1995, 37, 623–629. [Google Scholar] [CrossRef]
- Austin, J.; Singhal, N.; Voigt, R.; Smaill, F.; Gill, M.J.; Walmsley, S.; Salit, I.; Gilmour, J.; Schlech, W.F., 3rd; Choudhri, S.; et al. A community randomized controlled clinical trial of mixed carotenoids and micronutrient supplementation of patients with acquired immunodeficiency syndrome. Eur. J. Clin. Nutr. 2006, 60, 1266–1276. [Google Scholar] [CrossRef]
- Fawzi, W.W.; Msamanga, G.I.; Spiegelman, D.; Wei, R.; Kapiga, S.; Villamor, E.; Mwakagile, D.; Mugusi, F.; Hertzmark, E.; Essex, M.; et al. A randomized trial of multivitamin supplements and hiv disease progression and mortality. N. Engl. J. Med. 2004, 351, 23–32. [Google Scholar]
- Foster, B.; Francovic, A.; Drouin, C.; Akhtar, H.; Cameron, W. Trans-b-carotene and Retinoids can Effect Human Cytochrome p450 3a4-Mediated Metabolism (Abstract tupeb4564). In Proceedings of XIV International AIDS Conference, Barcelona, Spain, 7-12 July 2002.
- Ruhl, R.; Sczech, R.; Landes, N.; Pfluger, P.; Kluth, D.; Schweigert, F.J. Carotenoids and their metabolites are naturally occurring activators of gene expression via the pregnane x receptor. Eur. J. Nutr. 2004, 43, 336–343. [Google Scholar] [CrossRef]
- Kerr, B.; Yuen, G.; Daniels, R.; Quart, B.; Anderson, R. Strategic Approach to Nelfinavir Mesylate (nfv) Drug Interactions Involving cyp3a Metabolism (Abstract 429). In Proceedings of 4th Conference on Retroviruses and Opportunistic Infections, Washington, DC, USA, 22-26 January 1997.
- Zhang, K.E.; Wu, E.; Patick, A.K.; Kerr, B.; Zorbas, M.; Lankford, A.; Kobayashi, T.; Maeda, Y.; Shetty, B.; Webber, S. Circulating metabolites of the human immunodeficiency virus protease inhibitor nelfinavir in humans: Structural identification, levels in plasma, and antiviral activities. Antimicrob. Agents Chemother. 2001, 45, 1086–1093. [Google Scholar]
- Regazzi, M.; Maserati, R.; Villani, P.; Cusato, M.; Zucchi, P.; Briganti, E.; Roda, R.; Sacchelli, L.; Gatti, F.; Delle Foglie, P.; et al. Clinical pharmacokinetics of nelfinavir and its metabolite m8 in human immunodeficiency virus (hiv)-positive and HIV-hepatitis c virus-coinfected subjects. Antimicrob. Agents Chemother. 2005, 49, 643–649. [Google Scholar] [CrossRef]
- Meyers, D.G.; Maloley, P.A.; Weeks, D. Safety of antioxidant vitamins. Arch. Intern. Med. 1996, 156, 925–935. [Google Scholar] [CrossRef]
- van Heeswijk, R.P.; Khaliq, Y.; Gallicano, K.D.; Bourbeau, M.; Seguin, I.; Phillips, E.J.; Cameron, D.W. The pharmacokinetics of nelfinavir and m8 during pregnancy and post partum. Clin. Pharmacol. Ther. 2004, 76, 588–597. [Google Scholar] [CrossRef]
- Dimitrov, N.V.; Meyer, C.; Ullrey, E.; Chenoweth, W.; Michelakis, A.; Malone, W.; Boone, C.; Fink, G. Bioavailability of beta-carotene in humans. Am. J. Clin. Nutr. 1988, 48, 298–304. [Google Scholar]
- van Het Hof, K.H.; West, C.E.; Weststrate, J.A.; Hautvast, J.G. Dietary factors that affect the bioavailability of carotenoids. J. Nutr. 2000, 130, 503–506. [Google Scholar]
- Ribaya-Mercado, J.D.; Solon, F.S.; Solon, M.A.; Cabral-Barza, M.A.; Perfecto, C.S.; Tang, G.; Solon, J.A.; Fjeld, C.R.; Russell, R.M. Bioconversion of plant carotenoids to vitamin a in filipino school-aged children varies inversely with vitamin a status. Am. J. Clin. Nutr. 2000, 72, 455–465. [Google Scholar]
- Lemke, S.L.; Dueker, S.R.; Follett, J.R.; Lin, Y.; Carkeet, C.; Buchholz, B.A.; Vogel, J.S.; Clifford, A.J. Absorption and retinol equivalence of b-carotene in humans is influenced by dietary vitamin a intake. J. Lipid. Res. 2003, 44, 1591–1600. [Google Scholar] [CrossRef]
- Wang, X.D.; Marini, R.P.; Hebuterne, X.; Fox, J.G.; Krinsky, N.I.; Russell, R.M. Vitamin e enhances the lymphatic transport of beta-carotene and its conversion to vitamin a in the ferret. Gastroenterol 1995, 108, 719–726. [Google Scholar]
- Borel, P. Genetic variations involved in interindividual variability in carotenoid status. Mol. Nutr. Food Res. 2011, 55, 1–13. [Google Scholar] [CrossRef]
- Masimirembwa, C.; Bertilsson, L.; Johansson, I.; Hasler, J.A.; Ingelman-Sundberg, M. Phenotyping and genotyping of s-mephenytoin hydroxylase (cytochrome p450 2c19) in a shona population of zimbabwe. Clin. Pharmacol. Ther. 1995, 57, 656–661. [Google Scholar] [CrossRef]
- Persson, I.; Aklillu, E.; Rodrigues, F.; Bertilsson, L.; Ingelman-Sundberg, M. S-mephenytoin hydroxylation phenotype and cyp2c19 genotype among ethiopians. Pharmacogenetics 1996, 6, 521–526. [Google Scholar] [CrossRef]
- Goldstein, J.A.; Ishizaki, T.; Chiba, K.; de Morais, S.M.; Bell, D.; Krahn, P.M.; Evans, D.A. Frequencies of the defective cyp2c19 alleles responsible for the mephenytoin poor metabolizer phenotype in various oriental, caucasian, saudi arabian and american black populations. Pharmacogenetics 1997, 7, 59–64. [Google Scholar] [CrossRef]
- Haas, D.W.; Smeaton, L.M.; Shafer, R.W.; Robbins, G.K.; Morse, G.D.; Labbe, L.; Wilkinson, G.R.; Clifford, D.B.; D'Aquila, R.T.; De Gruttola, V.; et al. Pharmacogenetics of long-term responses to antiretroviral regimens containing efavirenz and/or nelfinavir: An adult aids clinical trials group study. J. Infect. Dis. 2005, 192, 1931–1942. [Google Scholar] [CrossRef]
- Khaliq, Y.; Gallicano, K.; Seguin, I.; Fyke, K.; Carignan, G.; Bulman, D.; Badley, A.; Cameron, D.W. Single and multiple dose pharmacokinetics of nelfinavir and cyp2c19 activity in human immunodeficiency virus-infected patients with chronic liver disease. Br. J. Clin. Pharmacol. 2000, 50, 108–115. [Google Scholar] [CrossRef]
- Droste, J.A.; Aarnoutse, R.E.; Koopmans, P.P.; Hekster, Y.A.; Burger, D.M. Evaluation of antiretroviral drug measurements by an interlaboratory quality control program. J. Acquir. Immune. Defic. Syndr. 2003, 32, 287–291. [Google Scholar] [CrossRef]
- Alexander, M.; Newmark, H.; Miller, R.G. Oral beta-carotene can increase the number of okt4+ cells in human blood. Immunol. Lett. 1985, 9, 221–224. [Google Scholar] [CrossRef]
- Watzl, B.; Bub, A.; Brandstetter, B.R.; Rechkemmer, G. Modulation of human t-lymphocyte functions by the consumption of carotenoid-rich vegetables. Br. J. Nutr. 1999, 82, 383–389. [Google Scholar]
- Chen, C.M.; Li, S.C.; Lin, Y.L.; Hsu, C.Y.; Shieh, M.J.; Liu, J.F. Consumption of purple sweet potato leaves modulates human immune response: T-lymphocyte functions, lytic activity of natural killer cell and antibody production. World J. Gastroenterol. 2005, 11, 5777–5781. [Google Scholar]
- Corridan, B.M.; O'Donoghue, M.; Hughes, D.A.; Morrissey, P.A. Low-dose supplementation with lycopene or beta-carotene does not enhance cell-mediated immunity in healthy free-living elderly humans. Eur. J. Clin. Nutr. 2001, 55, 627–635. [Google Scholar] [CrossRef]
- Murata, T.; Tamai, H.; Morinobu, T.; Manago, M.; Takenaka, H.; Hayashi, K.; Mino, M. Effect of long-term administration of beta-carotene on lymphocyte subsets in humans. Am. J. Clin. Nutr. 1994, 60, 597–602. [Google Scholar]
- Watson, R.R.; Prabhala, R.H.; Plezia, P.M.; Alberts, D.S. Effect of beta-carotene on lymphocyte subpopulations in elderly humans: Evidence for a dose-response relationship. Am. J. Clin. Nutr. 1991, 53, 90–94. [Google Scholar]
- Omenn, G.S.; Goodman, G.E.; Thornquist, M.D.; Balmes, J.; Cullen, M.R.; Glass, A.; Keogh, J.P.; Meyskens, F.L.; Valanis, B.; Williams, J.H.; et al. Effects of a combination of beta carotene and vitamin a on lung cancer and cardiovascular disease. N. Engl. J. Med. 1996, 334, 1150–1155. [Google Scholar] [CrossRef]
- Akhtar, M.; Bryan, M. Extraction and quantification of major carotenoids in processed foods and supplements by liquid chromatography. Food Chem. 2008, 111, 255–261. [Google Scholar] [CrossRef]
- Baede-van Dijk, P.A.; Hugen, P.W.; Verweij-van Wissen, C.P.; Koopmans, P.P.; Burger, D.M.; Hekster, Y.A. Analysis of variation in plasma concentrations of nelfinavir and its active metabolite m8 in hiv-positive patients. AIDS 2001, 15, 991–998. [Google Scholar] [CrossRef]
- Droste, J.A.; Verweij-Van Wissen, C.P.; Burger, D.M. Simultaneous determination of the hiv drugs indinavir, amprenavir, saquinavir, ritonavir, lopinavir, nelfinavir, the nelfinavir hydroxymetabolite m8, and nevirapine in human plasma by reversed-phase high-performance liquid chromatography. Ther. Drug Monit. 2003, 25, 393–399. [Google Scholar] [CrossRef]
- Piscitelli, S.C.; Rodvold, K.A.; Pai, M.P. Drug Interactions in Infectious Diseases, 3rd ed; Humana Press: Totowa, NJ, USA, 2011. [Google Scholar]
- FDA, Department of Health and Human Services, Guidance for Industry-In vivo Drug Metabolism / Drug Interaction Studies-Study Design, Data Analysis, and Recommendations for Dosing and Labeling. FDA: Rockville, MD, USA, 1999; pp. 1–16.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sheehan, N.L.; Heeswijk, R.P.G.v.; Foster, B.C.; Akhtar, H.; Singhal, N.; Seguin, I.; DelBalso, L.; Bourbeau, M.; Chauhan, B.M.; Boulassel, M.-R.; et al. The Effect of β-Carotene Supplementation on the Pharmacokinetics of Nelfinavir and Its Active Metabolite M8 in HIV-1-infected Patients. Molecules 2012, 17, 688-702. https://doi.org/10.3390/molecules17010688
Sheehan NL, Heeswijk RPGv, Foster BC, Akhtar H, Singhal N, Seguin I, DelBalso L, Bourbeau M, Chauhan BM, Boulassel M-R, et al. The Effect of β-Carotene Supplementation on the Pharmacokinetics of Nelfinavir and Its Active Metabolite M8 in HIV-1-infected Patients. Molecules. 2012; 17(1):688-702. https://doi.org/10.3390/molecules17010688
Chicago/Turabian StyleSheehan, Nancy L., Rolf P. G. van Heeswijk, Brian C. Foster, Humayoun Akhtar, Neera Singhal, Isabelle Seguin, Lina DelBalso, Marc Bourbeau, Bobby M. Chauhan, Mohammed-Rachid Boulassel, and et al. 2012. "The Effect of β-Carotene Supplementation on the Pharmacokinetics of Nelfinavir and Its Active Metabolite M8 in HIV-1-infected Patients" Molecules 17, no. 1: 688-702. https://doi.org/10.3390/molecules17010688