Effects of Yeast Polysaccharide on Growth and Flavonoid Accumulation in Fagopyrum tataricum Sprout Cultures
Abstract
:1. Introduction
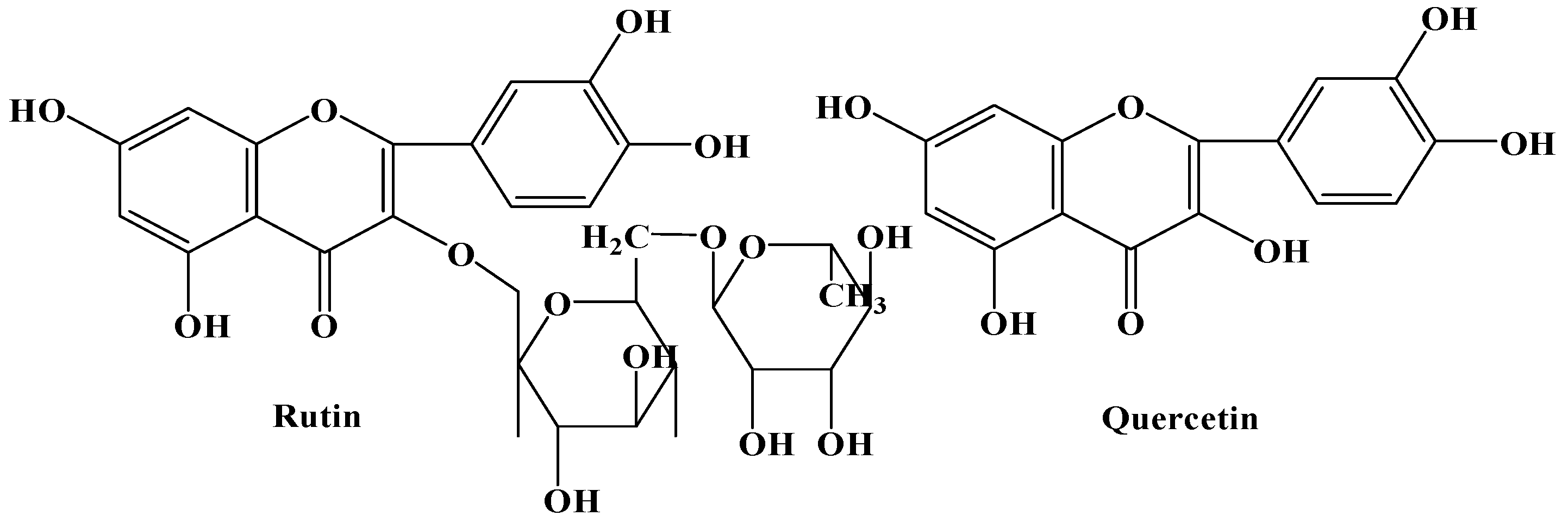
2. Results and Discussion
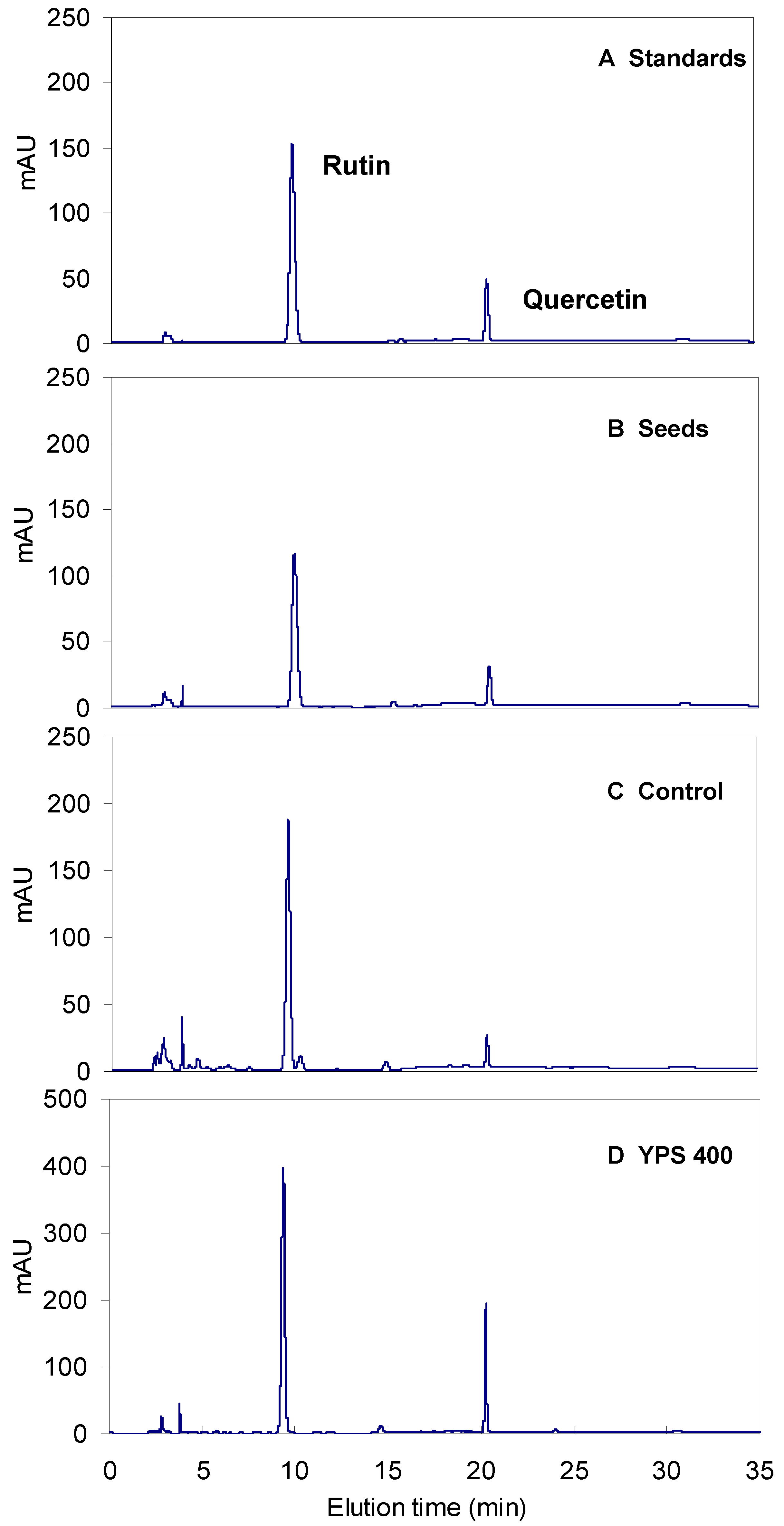
2.1. Sprout Growth and Flavonoids Accumulation of F. tataricum

2.2. Effects of YPS on F. tataricum Sprout Growth and Flavonoids Production

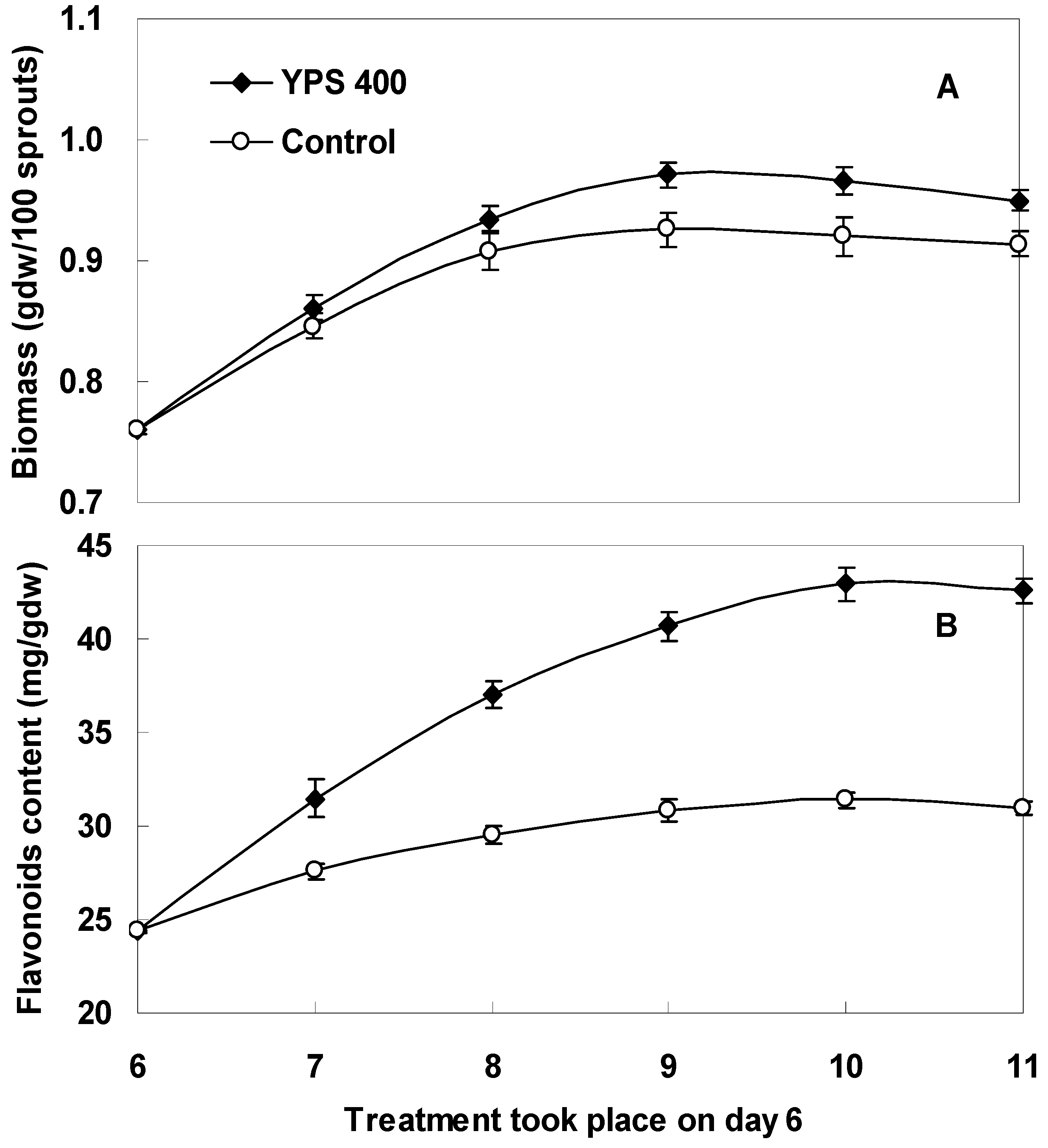
2.3. Kinetics of F. tataricum Sprout Growth and Flavonoid Accumulation after Treatment with YPS

3. Experimental
3.1. Source and Cultivation of Buckwheat Sprouts
3.2. Preparation and Application of YPS
3.3. Measurement of Biomass and Flavonoids Content
3.4. Measurement of PAL Activity
3.5. Statistical Analysis
4. Conclusions
Acknowledgements
References
- Fabjan, N.; Rode, J.; Košir, I.J.; Wang, Z.H.; Zhang, Z.; Kreft, I. Tartary buckwheat (Fagopyrum tataricum Gaertn.) as a source of dietary rutin and quercitrin. J. Agric. Food Chem. 2003, 51, 6452–6455. [Google Scholar]
- Guo, X.D.; Ma, Y.J.; Parry, J.; Gao, J.M.; Yu, L.L.; Wang, M. Phenolics content and antioxidant activity of tartary buckwheat from different locations. Molecules 2011, 16, 9850–9867. [Google Scholar] [CrossRef]
- Zhao, G.; Zou, L.; Wang, Z.G.; Hu, H.L.; Hu, Y.B.; Peng, L.X. Pharmacokinetic profile of total quercetin after single oral dose of tartary buckwheat extracts in rats. J. Agric. Food Chem. 2011, 59, 4435–4441. [Google Scholar]
- Siatka, T.; Kašparová, M. Seasonal variation in total phenolic and flavonoid contents and DPPH scavenging activity of Bellis perennis L. flowers. Molecules 2010, 15, 9450–9461. [Google Scholar] [CrossRef]
- Verardo, V.; Arráez-Román, D.; Segura-Carretero, A.; Marconi, E.; Fernández-Gutiérrez, A.; Caboni, M.F. Identification of buckwheat phenolic compounds by reverse phase high performance liquid chromatography-electrospray ionization-time of flight-mass spectrometry (RP-HPLC-ESI-TOF-MS). J. Cereal Sci. 2010, 52, 170–176. [Google Scholar] [CrossRef]
- Khadem, S.; Marles, R.J. Chromone and flavonoid alkaloids: Occurrence and bioactivity. Molecules 2012, 17, 191–206. [Google Scholar] [CrossRef]
- Zielińska, D.; Turemko, M.; Kwiatkowski, J.; Zieliński, H. Evaluation of flavonoid contents and antioxidant capacity of the aerial parts of common and tartary buckwheat plants. Molecules 2012, 17, 9668–9682. [Google Scholar] [CrossRef]
- Ikeda, K. Buckwheat: Composition, Chemistry, and Processing. Adv. Food. Nutr. Res. 2002, 44, 395–434. [Google Scholar] [CrossRef]
- Zhao, G. Buckwheat Processing and New Product Development Technology; Beijing Science and Technology Publisher: Beijing, China, 2010; pp. 180–185. [Google Scholar]
- Qin, P.Y.; Ma, T.J.; Wu, L.; Shan, F.; Ren, G.X. Identification of tartary buckwheat tea aroma compounds with gas chromatography-mass spectrometry. J. Food Sci. 2011, 76, s401–s407. [Google Scholar] [CrossRef]
- Kim, S.L.; Kim, S.K.; Park, C.H. Introduction and nutritional evaluation of buckwheat sprouts as a new vegetable. Food Res. Int. 2004, 37, 319–327. [Google Scholar] [CrossRef]
- Maejima, Y.; Nakatsugawa, H.; Ichida, D.; Maejima, M.; Aoyagi, Y.; Maoka, T.; Etoh, H. Functional compounds in fermented buckwheat sprouts. Biosci. Biotechnol. Biochem. 2011, 75, 1708–1712. [Google Scholar] [CrossRef]
- Lin, L.Y.; Peng, C.C.; Yang, Y.L.; Peng, R.Y. Optimization of bioactive compounds in buckwheat sprouts and their effect on blood cholesterol in hamsters. J. Agric. Food Chem. 2008, 56, 1216–1223. [Google Scholar] [CrossRef]
- Peng, C.C.; Chen, K.C.; Yang, Y.L.; Lin, L.Y.; Peng, R.Y. Aqua-culture improved buckwheat sprouts with more abundant precious nutrients and hypolipidemic activity. Int. J. Food Sci. Nutr. 2009, 60, 232–245. [Google Scholar]
- Kim, H.J.; Park, K.J.; Lim, J.H. Metabolomic analysis of phenolic compounds in buckwheat (Fagopyrum esculentum M.) sprouts treated with methyl jasmonate. J. Agric. Food Chem. 2011, 59, 5707–5713. [Google Scholar]
- Chong, T.M.; Abdullah, M.A.; Lai, Q.M.; Nor’Aini, F.M.; Lajis, N.H. Effective elicitation factors in Morinda elliptica cell suspension culture. Proc. Biochem. 2005, 40, 3397–3405. [Google Scholar] [CrossRef]
- Zhou, L.G.; Wu, J.Y. Development and application of medicinal plant tissue cultures for production of drugs and herbal medicinals in China. Nat. Prod. Rep. 2006, 23, 789–810. [Google Scholar] [CrossRef]
- Smetanska, I. Production of secondary metabolites using plant cell cultures. Adv. Biochem. Eng. Biotechnol. 2008, 111, 187–228. [Google Scholar]
- Chen, H.; Chen, F. Effect of yeast elicitor on the secondary metabolism of Ti-transformed Salvia miltiorrhiza cell suspension cultures. Plant Cell Rep. 2000, 19, 710–717. [Google Scholar] [CrossRef]
- Putalun, W.; Luealon, W.; De-Eknamkul, W.; Tanaka, H. Improvement of artemisinin production by chitosan in hairy root cultures of Artemisia annua L. Biotechnol. Lett. 2007, 29, 1143–1146. [Google Scholar] [CrossRef]
- Prakash, G.; Srivastava, A.K. Statistical elicitor optimization studies for the enhancement of azadirachtin production in bioreactor Azadirachta indica cell cultivation. Biochem. Eng. J. 2008, 40, 218–226. [Google Scholar] [CrossRef]
- Putalun, W.; Udomsin, O.; Yusakul, G.; Juengwatanatrakul, T.; Sakamoto, S.; Tanaka, H. Enhanced plumbagin production from in vitro cultures of Drosera burmanii using elicitation. Biotechnol. Lett. 2010, 32, 721–724. [Google Scholar] [CrossRef]
- Zhao, J.; Zhou, L.; Wu, J. Effects of biotic and abiotic elicitors on cell growth and tanshinone accumulation in Salvia miltiorrhiza cell cultures. Appl. Microbiol. Biotechnol. 2010, 87, 137–144. [Google Scholar] [CrossRef]
- Liu, J.F.; Li, X.Y.; Meng, R. Preliminary studies on the factors for promoting flavonoids production during the germination process of tatary buckwheat. Sci. Technol. Food Ind. 2006, 27, 106–108. [Google Scholar]
- Suzuki, T.; Honda, Y.; Mukasa, Y. Effects of UV-B radiation, cold and desiccation stress on rutin concentration and rutin glucosidase activity in tartary buckwheat (Fagopyrum tataricum) leaves. Plant Sci. 2005, 168, 1303–1307. [Google Scholar] [CrossRef]
- Breznik, B.; Germ, M.; Gaberščik, A.; Kreft, I. Combined effects of elevated UV-B radiation and the addition of selenium on common (Fagopyrum esculentum Moench) and tartary [Fagapyrum tataricum (L.) Gaertn.] buckwheat. Photosynthetica 2005, 43, 583–589. [Google Scholar]
- Leung, P.H.; Zhao, S.N.; Ho, K.P.; Wu, J.Y. Chemical properties and antioxidant activity of exopolysaccharides from mycelial culture of Cordyceps sinensis fungus Cs-HK1. Food Chem. 2009, 114, 1251–1256. [Google Scholar] [CrossRef]
- Wu, J.Y.; Lin, L.D. Ultrasound-induced stress responses of Panax ginseng cells: Enzymatic browning and phenolics production. Biotechnol. Prog. 2002, 18, 862–866. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the buckwheat sprouts are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhao, G.; Zhao, J.; Peng, L.; Zou, L.; Wang, J.; Zhong, L.; Xiang, D. Effects of Yeast Polysaccharide on Growth and Flavonoid Accumulation in Fagopyrum tataricum Sprout Cultures. Molecules 2012, 17, 11335-11345. https://doi.org/10.3390/molecules171011335
Zhao G, Zhao J, Peng L, Zou L, Wang J, Zhong L, Xiang D. Effects of Yeast Polysaccharide on Growth and Flavonoid Accumulation in Fagopyrum tataricum Sprout Cultures. Molecules. 2012; 17(10):11335-11345. https://doi.org/10.3390/molecules171011335
Chicago/Turabian StyleZhao, Gang, Jianglin Zhao, Lianxin Peng, Liang Zou, Jingbo Wang, Lingyun Zhong, and Dabing Xiang. 2012. "Effects of Yeast Polysaccharide on Growth and Flavonoid Accumulation in Fagopyrum tataricum Sprout Cultures" Molecules 17, no. 10: 11335-11345. https://doi.org/10.3390/molecules171011335




