Pumpkin (Cucurbita moschata) Fruit Extract Improves Physical Fatigue and Exercise Performance in Mice
Abstract
:1. Introduction
2. Results and Discussion
2.1. Body Weight, Skeletal Muscle Mass and Liver Weight
| Characteristics | Vehicle | CME-50 | CME-100 | CME-250 |
|---|---|---|---|---|
| Initial BW (g) | 37.6 ± 0.6 | 37.7 ± 0.6 | 36.9 ± 0.4 | 36.9 ± 0.9 |
| Final BW (g) | 38.5 ± 0.3 | 40.4 ± 0.6 | 39.1 ± 0.6 | 39.0 ± 1.0 |
| Skeletal muscle (g) | 0.35 ± 0.01 | 0.37 ± 0.01 | 0.36 ± 0.01 | 0.36 ± 0.01 |
| Liver (g) | 2.00 ± 0.05 | 2.17 ± 0.08 | 2.00 ± 0.03 | 2.07 ± 0.09 |
2.2. Effect of C. moschata Fruit Extract on the Clinical Biochemistry Tests
| Parameters | Vehicle | CME-50 | CME-100 | CME-250 |
|---|---|---|---|---|
| AST (U/L) | 83.9 ± 6.2 | 70.4 ± 4.4 | 83.0 ± 5.0 | 76.3 ± 8.5 |
| ALT (U/L) | 48.0 ± 2.8 | 45.6 ± 5.1 | 49.9 ± 2.8 | 43.9 ± 4.0 |
| ALP (U/L) | 120 ± 14 | 128 ± 16 | 137 ± 20 | 124 ± 13 |
| LDH (U/L) | 425 ± 34 | 429 ± 33 | 425 ± 31 | 362 ± 44 |
| Albumin (g/dL) | 2.7 ± 0.1 | 2.7 ± 0.1 | 2.8 ± 0.1 | 2.9 ± 0.1 |
| TBIL(μg/dL) | 55.2 ± 9.1 | 56.9 ± 5.6 | 59.6 ± 5.0 | 52.4 ± 3.2 |
| TP (g/dL) | 5.1 ± 0.1 | 5.1 ± 0.2 | 5.2 ± 0.2 | 5.2 ± 0.1 |
| BUN (mg/dL) | 25.5 ± 1.8 b | 20.7 ± 2.2 a | 21.0 ± 0.9 a | 19.7 ± 0.8 a |
| Creatinine (mg/dL) | 0.29 ± 0.01 | 0.26 ± 0.02 | 0.28 ± 0.01 | 0.28 ± 0.01 |
| UA (mg/dL) | 1.3 ± 0.1 | 1.1 ± 0.1 | 1.0 ± 0.1 | 1.1 ± 0.1 |
| TC (mg/dL) | 107 ± 6 | 110 ± 12 | 118 ± 5 | 107 ± 8 |
| TG (mg/dL) | 73.4 ± 10.5 | 71.3 ± 8.0 | 65.3 ± 5.9 | 60.0 ± 6.0 |
2.3. Effect of C. moschata Fruit Extract on Forelimb Grip Strength


2.4. Effect of C. moschata Fruit Extract on Exercise Performance in a Weight-Loaded Swimming Test

2.5. Effect of Supplement with C. moschata Fruit Extract on Plasma Lactate, Ammonia, Glucose and CK Levels after an Acute Exercise Challenge
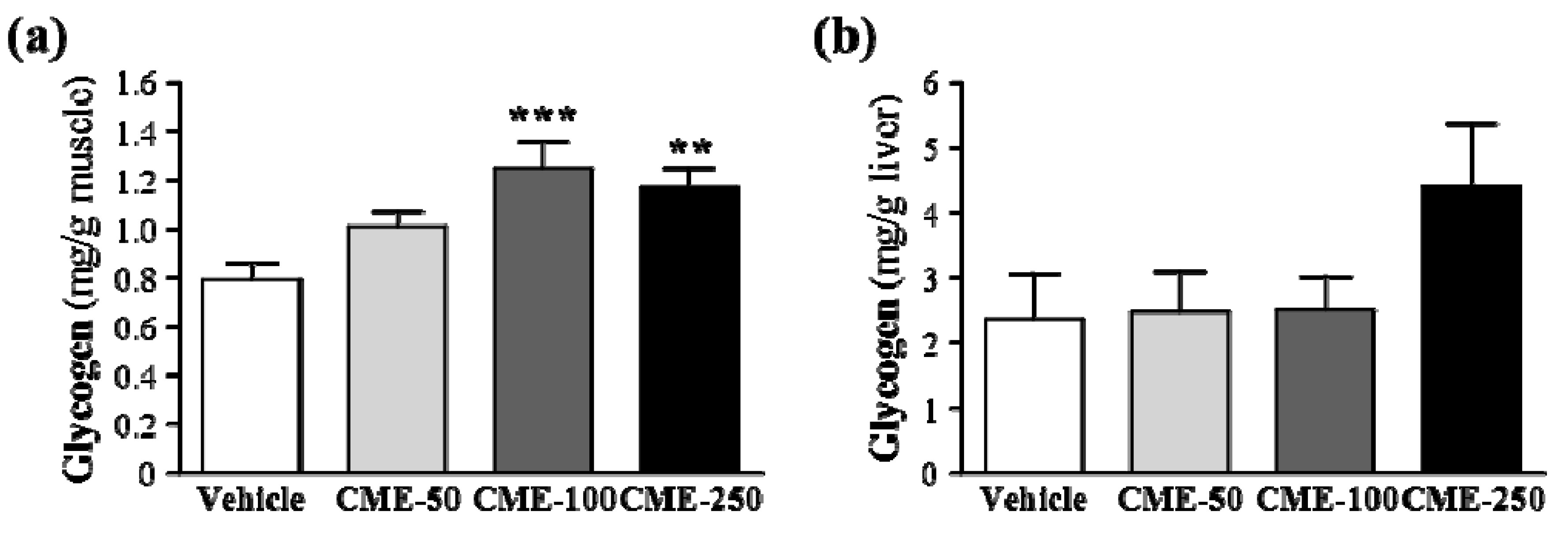
2.6. Effect of C. moschata Fruit Extract on Muscular and Hepatic Glycogen Levels
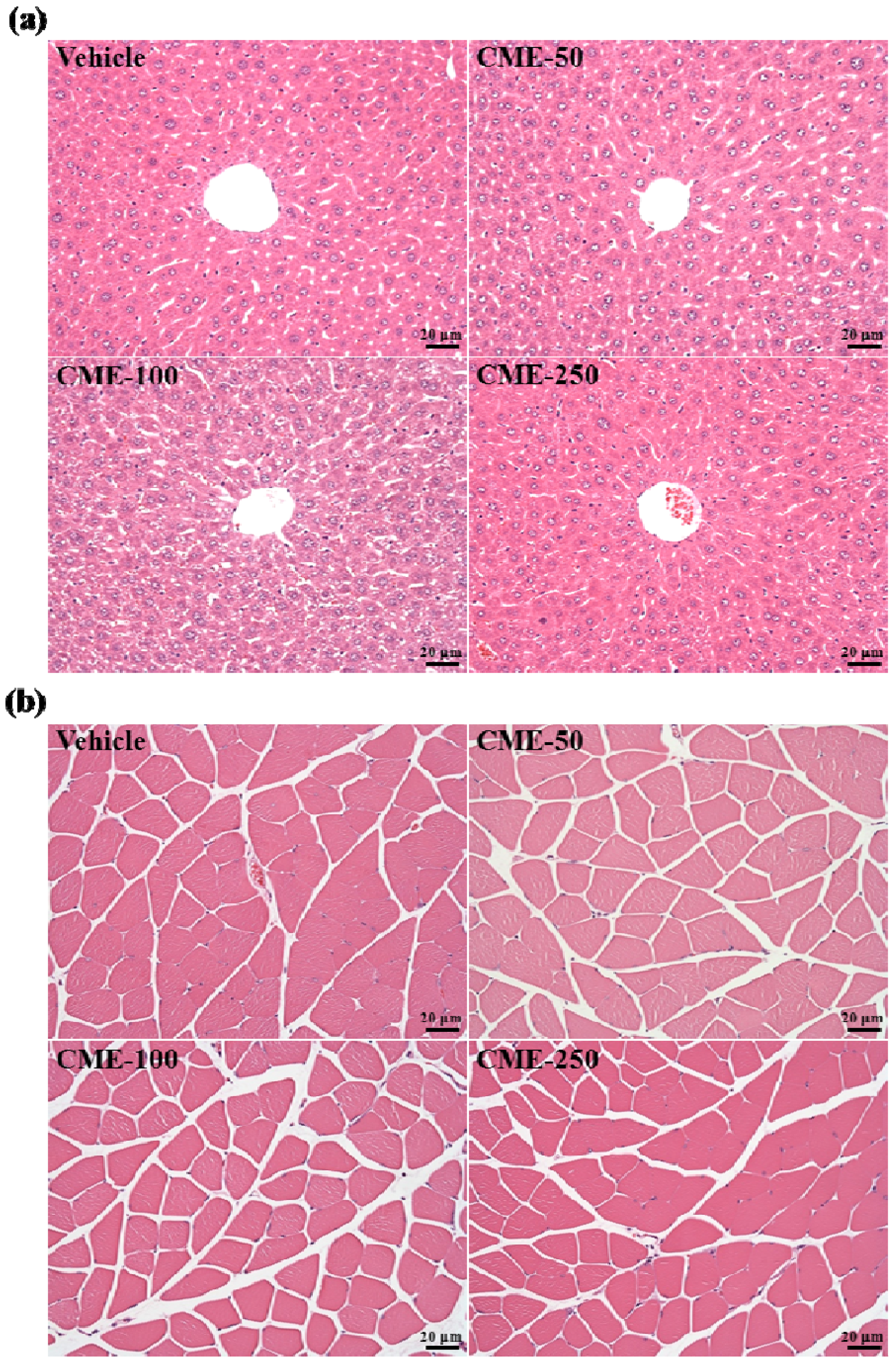
2.7. Effect of C. moschata Fruit Extract on Hepatic and Muscular Tissues
3. Experimental
3.1. Plant Material and Extraction
3.2. Animal and Experiment Design
3.3. Forelimb Grip Strength
3.4. Swimming Exercise Performance Test
3.5. Determination of Blood Biochemical Variables
3.6. Tissue Glycogen Determination
3.7. Histological Examination of Liver and Skeletal Muscle Tissues
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
Conflict of Interest
- Samples Availability: Samples of the ethanolic extracts of C . moschata are available from the authors.
References
- Kim, K.M.; Yu, K.W.; Kang, D.H.; Suh, H.J. Anti-stress and anti-fatigue effect of fermented rice bran. Phytother. Res. 2002, 16, 700–702. [Google Scholar] [CrossRef]
- Morihara, N.; Nishihama, T.; Ushijima, M.; Ide, N.; Takeda, H.; Hayama, M. Garlic as an anti-fatigue agent. Mol. Nutr. Food Res. 2007, 51, 1329–1334. [Google Scholar] [CrossRef]
- Hsu, M.C.; Chien, K.Y.; Hsu, C.C.; Chung, C.J.; Chan, K.H.; Su, B. Effects of BCAA, arginine and carbohydrate combined drink on post-exercise biochemical response and psychological condition. Chin. J. Physiol. 2011, 54, 71–78. [Google Scholar] [CrossRef]
- Liu, W.; Liu, W.L.; Liu, C.M.; Liu, J.H.; Yang, S.B.; Zheng, H.J.; Lei, H.W.; Ruan, R.; Li, T.; Tu, Z.C.; et al. Medium-chain fatty acid nanoliposomes for easy energy supply. Nutrition 2011, 27, 700–706. [Google Scholar] [CrossRef]
- Makni, M.; Fetoui, H.; Gargouri, N.K.; Garouiel, M.; Jaber, H.; Makni, J.; Boudawara, T.; Zeghal, N. Hypolipidemic and hepatoprotective effects of flax and pumpkin seed mixture rich in omega-3 and omega-6 fatty acids in hypercholesterolemic rats. Food Chem. Toxicol. 2008, 46, 3714–3720. [Google Scholar] [CrossRef]
- Jiang, Z.; Du, Q. Glucose-lowering activity of novel tetrasaccharide glyceroglycolipids from the fruits of Cucurbita moschata. Bioorg. Med. Chem. Lett. 2011, 21, 1001–1003. [Google Scholar] [CrossRef]
- Zhang, B.; Huang, H.; Xie, J.; Xu, C.; Chen, M.; Wang, C.; Yang, A.; Yin, Q. Cucurmosin induces apoptosis of BxPC-3 human pancreatic cancer cells via inactivation of the EGFR signaling pathway. Oncol. Rep. 2012, 27, 891–897. [Google Scholar]
- Lee, J.; Kim, D.; Choi, J.; Choi, H.; Ryu, J.H.; Jeong, J.; Park, E.J.; Kim, S.H.; Kim, S. Dehydrodiconiferyl alcohol isolated from Cucurbita moschata shows anti-adipogenic and anti-lipogenic effects in 3T3-L1 cells and primary mouse embryonic fibroblasts. J. Biol. Chem. 2012, 287, 8839–8851. [Google Scholar]
- Belluardo, N.; Westerblad, H.; Mudó, G.; Casabona, A.; Bruton, J.; Caniglia, G.; Pastoris, O.; Grassi, F.; Ibáñez, C.F. Neuromuscular junction disassembly and muscle fatigue in mice lacking neurotrophin-4. Mol. Cell. Neurosci. 2001, 18, 56–67. [Google Scholar] [CrossRef]
- Brancaccio, P.; Maffulli, N.; Buonauro, R.; Limongelli, F.M. Serum enzyme monitoring in sports medicine. Clin. Sports Med. 2008, 27, 1–18. [Google Scholar] [CrossRef]
- Cairns, S.P. Lactic acid and exercise performance: Culprit or friend? Sports Med. 2006, 36, 279–291. [Google Scholar] [CrossRef]
- Tashiro, S. Studies on alkaligenesis in tissues: I. Ammonia production in the nerve fiber during excitation. Am. J. Physiol. 1922, 60, 519–543. [Google Scholar]
- Carvalho-Peixoto, J.; Alves, R.C.; Cameron, L.C. Glutamine and carbohydrate supplements reduce ammonemia increase during endurance field exercise. Appl. Physiol. Nutr. Metab. 2007, 32, 1186–1190. [Google Scholar] [CrossRef]
- Suh, S.H.; Paik, I.Y.; Jacobs, K. Regulation of blood glucose homeostasis during prolonged exercise. Mol. Cells 2007, 23, 272–279. [Google Scholar]
- Warren, G.L.; Ingalls, C.P.; Lowe, D.A.; Armstrong, R.B. Excitation-contraction uncoupling: Major role in contraction-induced muscle injury. Exerc. Sport Sci. Rev. 2001, 29, 82–87. [Google Scholar] [CrossRef]
- Sahlin, K.; Tonkonogi, M.; Söderlund, K. Energy supply and muscle fatigue in humans. Acta Physiol. Scand. 1998, 162, 261–266. [Google Scholar] [CrossRef]
- Young, A.J.; Castellani, J.W. Exertion-induced fatigue and thermoregulation in the cold. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2001, 128, 769–776. [Google Scholar] [CrossRef]
- Huang, C.C.; Hsu, M.C.; Huang, W.C.; Yang, H.R.; Hou, C.C. Triterpenoid-rich extract from Antrodia camphorata improves physical fatigue and exercise performance in mice. Evid. Based Complement. Alternat. Med. 2012. [Google Scholar] [CrossRef]
- Chamberland, V.; Rioux, P. Not only students can express alcohol dehydrogenase: Goldfish can too! Adv. Physiol. Educ. 2010, 34, 222–227. [Google Scholar] [CrossRef]
- Ho, T.J.; Huang, C.C.; Huang, C.Y.; Lin, W.T. Fasudil, a Rho-kinase inhibitor, protects against excessive endurance exercise training-induced cardiac hypertrophy, apoptosis and fibrosis in rats. Eur. J. Appl. Physiol. 2012, 112, 2943–2955. [Google Scholar] [CrossRef]
- Kim, M.Y.; Kim, E.J.; Kim, Y.N.; Choi, C.; Lee, B.H. Comparison of the chemical compositions and nutritive values of various pumpkin (Cucurbitaceae) species and parts. Nutr. Res. Pract. 2012, 6, 21–27. [Google Scholar] [CrossRef]
- Hadad, N.; Levy, R. The synergistic anti-inflammatory effects of lycopene, lutein, β-carotene, and carnosic acid combinations via redox-based inhibition of NF-κB signaling. Free Radic. Biol. Med. 2012, 53, 1381–1391. [Google Scholar] [CrossRef]
- Mueller, L.; Boehm, V. Antioxidant activity of β-carotene compounds in different in vitro assays. Molecules 2011, 16, 1055–1069. [Google Scholar] [CrossRef]
- Tanaka, T.; Shnimizu, M.; Moriwaki, H. Cancer chemoprevention by carotenoids. Molecules 2012, 17, 3202–3242. [Google Scholar] [CrossRef]
- Umigai, N.; Tanaka, J.; Tsuruma, K.; Shimazawa, M.; Hara, H. Crocetin, a carotenoid derivative, inhibits VEGF-induced angiogenesis via suppression of p38 phosphorylatio. Curr. Neurovasc. Res. 2012, 9, 102–109. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, S.-Y.; Huang, W.-C.; Liu, C.-C.; Wang, M.-F.; Ho, C.-S.; Huang, W.-P.; Hou, C.-C.; Chuang, H.-L.; Huang, C.-C. Pumpkin (Cucurbita moschata) Fruit Extract Improves Physical Fatigue and Exercise Performance in Mice. Molecules 2012, 17, 11864-11876. https://doi.org/10.3390/molecules171011864
Wang S-Y, Huang W-C, Liu C-C, Wang M-F, Ho C-S, Huang W-P, Hou C-C, Chuang H-L, Huang C-C. Pumpkin (Cucurbita moschata) Fruit Extract Improves Physical Fatigue and Exercise Performance in Mice. Molecules. 2012; 17(10):11864-11876. https://doi.org/10.3390/molecules171011864
Chicago/Turabian StyleWang, Shih-Yi, Wen-Ching Huang, Chieh-Chung Liu, Ming-Fu Wang, Chin-Shan Ho, Wen-Pei Huang, Chia-Chung Hou, Hsiao-Li Chuang, and Chi-Chang Huang. 2012. "Pumpkin (Cucurbita moschata) Fruit Extract Improves Physical Fatigue and Exercise Performance in Mice" Molecules 17, no. 10: 11864-11876. https://doi.org/10.3390/molecules171011864





