Synthesis and Antiplasmodial Activity of Betulinic Acid and Ursolic Acid Analogues
Abstract
:1. Introduction
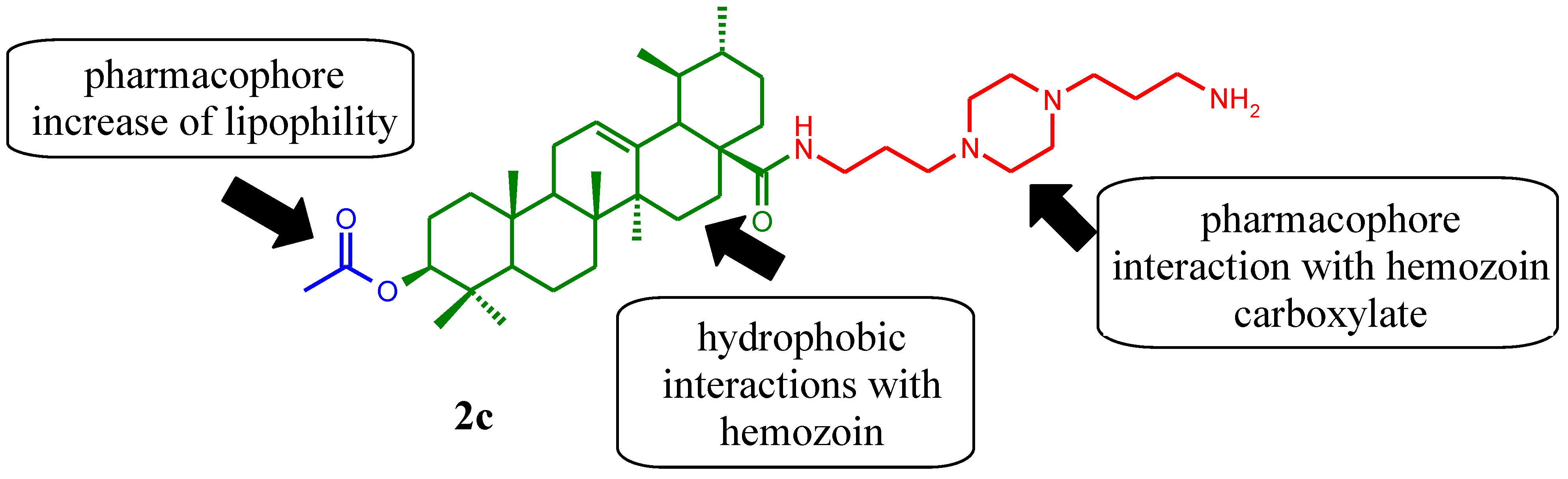
2. Results and Discussion
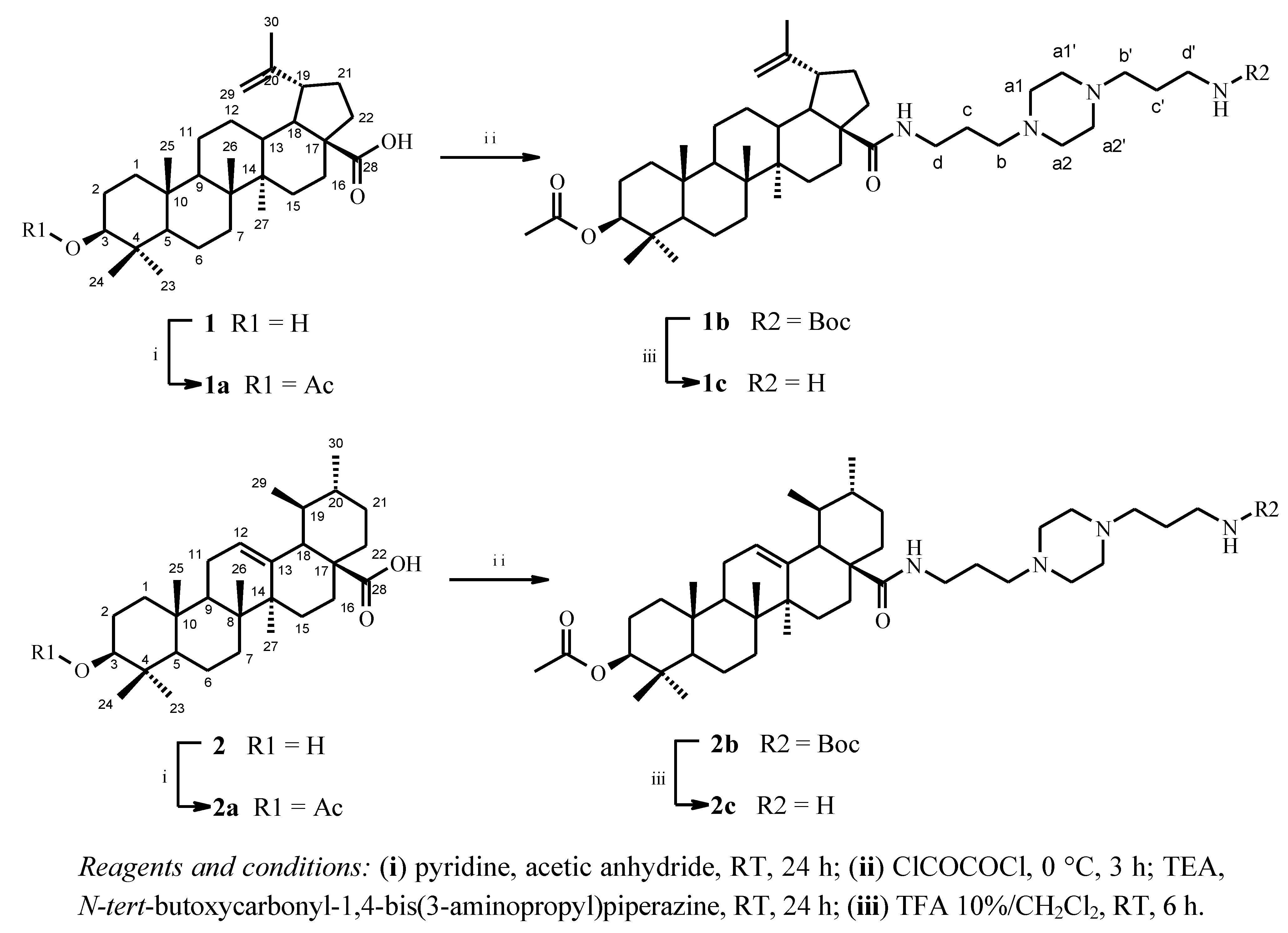
2.1. Chemical Synthesis
2.2. Antiplasmodial Assay
2.3. Cytotoxicity Assay
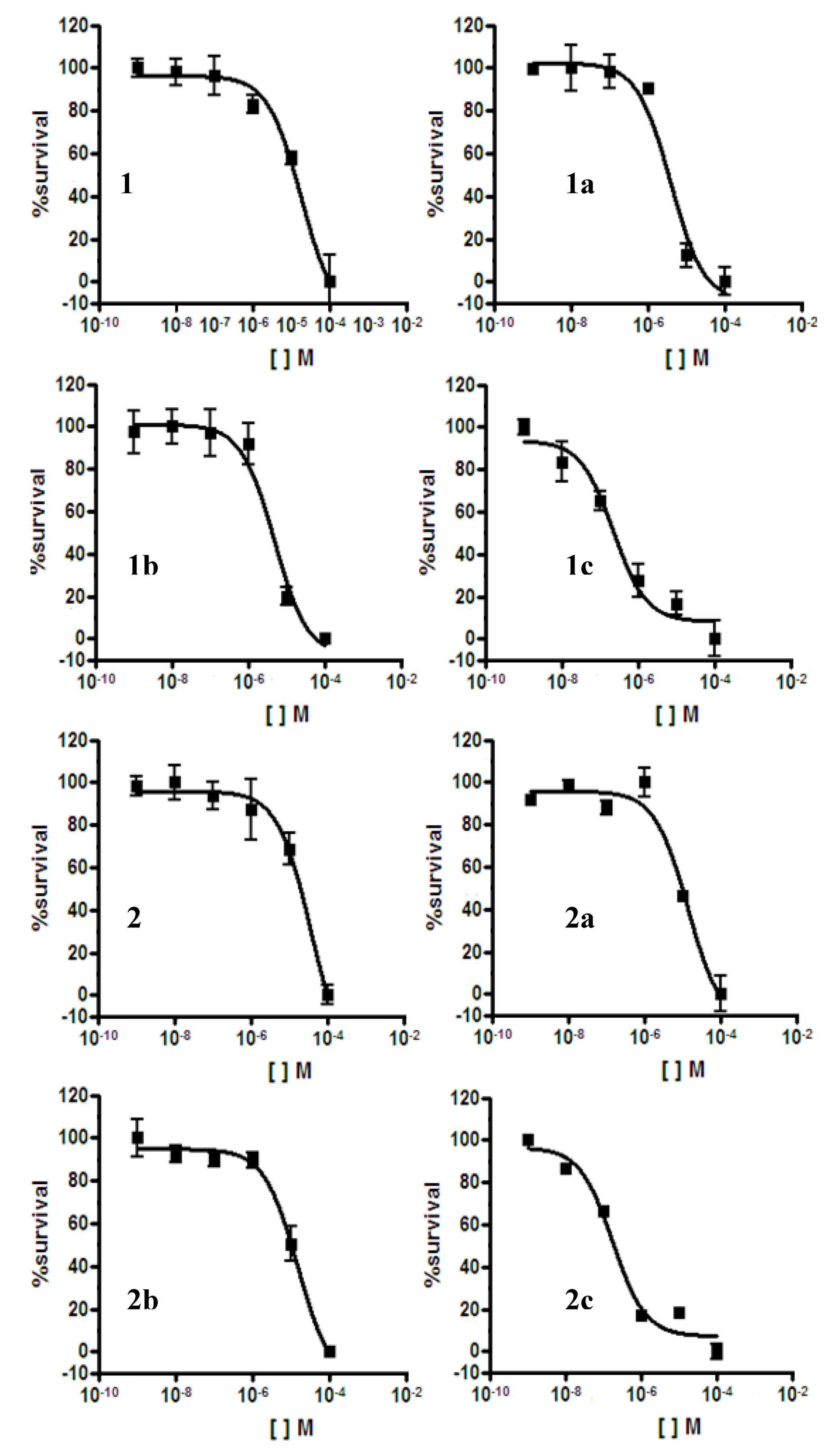
| Compounds | IC50 (µM) P. falciparum 3D7 | IC50 (µM) HEK293T | Selectivity Index (SI) 48 h | |
|---|---|---|---|---|
| 24 h | 48 h | |||
| 1 | 18 | - | - | - |
| 1a | 4 | >100 | >100 | - |
| 1b | 5 | 4 | 4 | 0.8 |
| 1c | 0.220 | 4 | 4 | 18 |
| 2 | 36 | - | - | - |
| 2a | 14 | - | - | - |
| 2b | 15 | - | - | - |
| 2c | 0.175 | 4 | 4 | 23 |
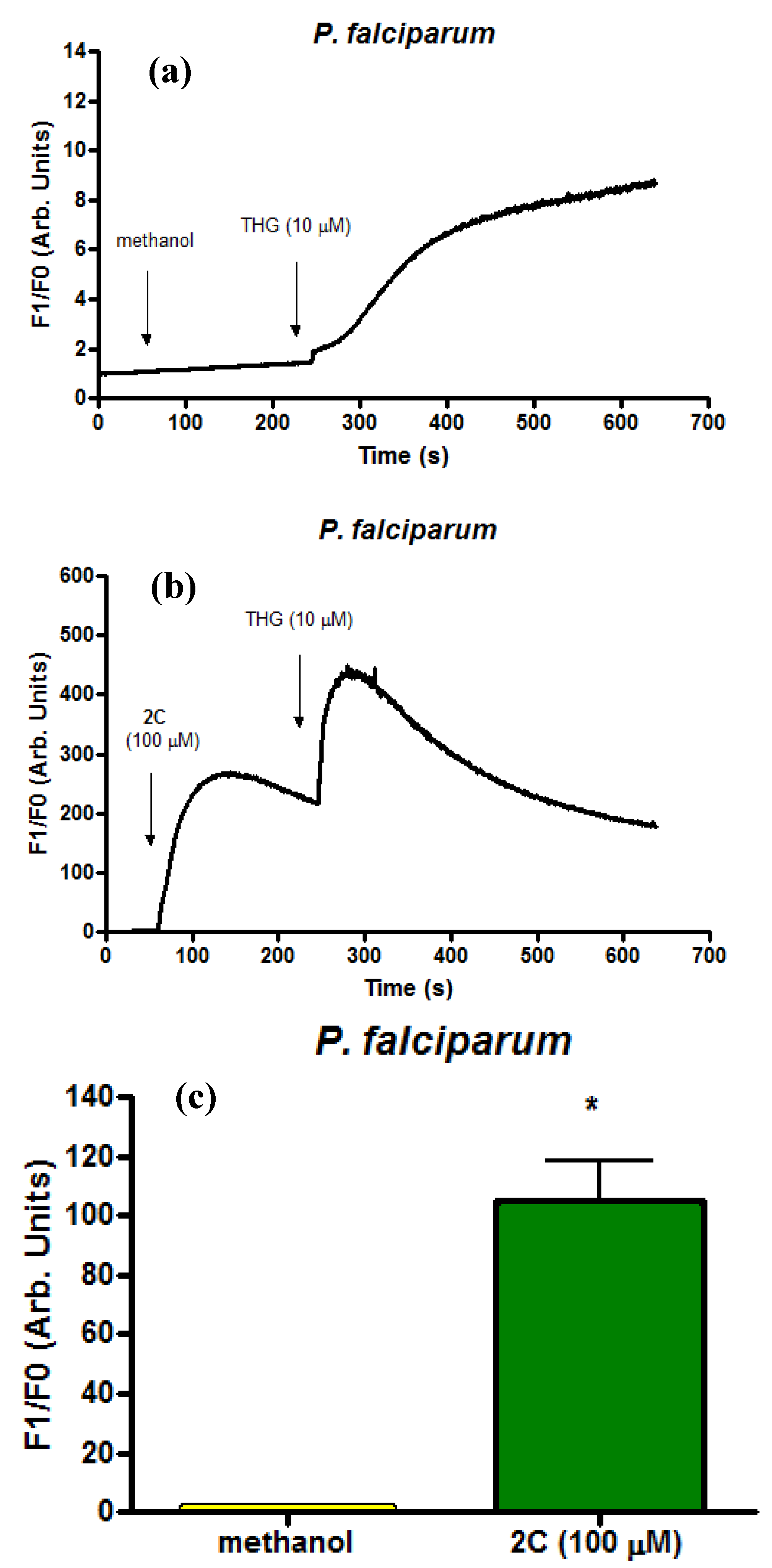
2.4. Infected Erythrocytes: Loading with the Calcium Indicator Fluo4/AM
3. Experimental
3.1. General
3.2. Plant Material and Isolation of Triterpenes
3.3. Chemical Synthesis
3.4. Antiplasmodial Assay
3.5. Cytotoxicity Assay
3.6. Infected Erythrocytes: Loading with the Calcium Indicator Fluo4/AM
3.7. Statistical Analysis
4. Conclusions
Acknowledgements
- Sample Availability: Samples of the compounds are available from the authors.
References
- WHO. World Malaria Report. 2011. Available online: http://www.who.int/malaria/world_malaria_report_2011/en/ (accessed on 11 August 2012).
- Mita, T.; Tanabe, K.; Kita, K. Spread and evolution of Plasmodium falciparum drug resistance. Parasitol. Int. 2009, 58, 201–209. [Google Scholar] [CrossRef]
- White, N.J. Qinghaosu (artemisinin): The price of success. Science 2008, 320, 330–334. [Google Scholar] [CrossRef]
- Frearson, J.A.; Wyatt, P.G.; Gilbert, I.H.; Fairlamb, A.H. Target assessment for antiparasitic drug discovery. Trends Parasitol. 2007, 23, 589–595. [Google Scholar] [CrossRef]
- Krettli, A.U.; Adebayo, J.O.; Krettli, L.G. Testing of natural products and synthetic molecules aiming at new antimalarials. Curr. Drug Targets 2009, 10, 261–270. [Google Scholar] [CrossRef]
- Steele, J.C.P.; Warhurst, D.C.; Kirby, G.C.; Simmonds, M.S.J. In vitro and in vivo evaluation of betulinic acid as an antimalarial. Phytother. Res. 1999, 13, 115–119. [Google Scholar] [CrossRef]
- Ziegler, H.L.; Franzyk, H.; Sairafianpour, M.; Tabatabai, M.; Tehrani, M.D.; Bagherzadeh, K. Erythrocyte membrane modifying agents and the inhibition of Plasmodium falciparum growth: Structure-activity relationships for betulinic acid analogues. Bioorg. Med. Chem. 2004, 12, 119–127. [Google Scholar] [CrossRef]
- Liu, J. Oleanolic acid and ursolic acid: Research perspectives. J. Ethnopharmacol. 2005, 100, 92–94. [Google Scholar] [CrossRef]
- Yogeeswari, P.; Sriram, D. Betulinic acid and its derivatives: A review on their biological properties. Curr. Med. Chem. 2005, 12, 657–666. [Google Scholar] [CrossRef]
- Gnoatto, S.C.B.; Susplugas, S.; Dalla Vechia, L.; Ferreira, T.B.; Dassonville-Klimpt, A.; Zimmer, K.R.; Demailly, C.; Da Nascimento, S.; Guillon, J.; Grellier, P.; et al. Pharmacomodulation on the 3-acetylursolic acid skeleton: Design, Synthesis, And biological evaluation of novel N-{3-[4-(3-aminopropyl)piperazinyl]propyl}-3-O-acetylursolamide derivatives as antimalarial agents. Bioorg. Med. Chem. 2008, 16, 771–782. [Google Scholar] [CrossRef]
- Girault, S.; Grellier, P.; Berecibar, A.; Maes, L.; Lemiere, P.; Mouray, E. Antiplasmodial activity and cytotoxicity of bis-, tris-, and tetraquinolines with linear or cyclic amino linkers. J. Med. Chem. 2001, 44, 1658–1665. [Google Scholar] [CrossRef]
- Ryckebusch, A.; Deprez-Poulain, R.; Debreu-Fontaine, M.A.; Vandaele, R.; Mouray, E.; Grellier, P. Parallel synthesis and anti-malarial activity of a sulfonamide library. Bioorg. Med. Chem. Lett. 2002, 12, 2595–2598. [Google Scholar] [CrossRef]
- Beraldo, F.H.; Almeida, F.M.; da Silva, A.M.; Garcia, C.R.S. Cyclic AMP and calcium interplay as second messengers in melatonin-dependent regulation of Plasmodium falciparum cell cycle. J.Cell Biol. 2005, 170, 551–557. [Google Scholar] [CrossRef]
- Beraldo, F.H.; Mikoshiba, K.; Garcia, C.R.S. Human malarial parasite, Plasmodium falciparum, displays capacitative calcium entry: 2-Aminoethyl diphenylborinate blocks the signal transduction pathway of melatonin action on the P. falciparum cell cycle. J. Pineal Res. 2007, 43, 360–364. [Google Scholar] [CrossRef]
- Garcia, C.R.S.; Azevedo, M.F.; Wunderlich, G.; Budu, A.; Young, J.A.; Bannister, L. Plasmodium in the postgenomic era: New insights into the molecular cell biology of malaria parasites. Int. Rev. Cell Mol. Biol. 2008, 266, 85–156. [Google Scholar] [CrossRef]
- Koyama, F.C.; Chakrabarti, D.; Garcia, C.R.S. Molecular machinery of signal transduction and cell cycle regulation in Plasmodium. Mol. Biochem. Parasitol. 2009, 165, 1–7. [Google Scholar] [CrossRef]
- Traore-Keita, F.; Gasquet, M.; Di Giorgio, C.; Ollivier, E.; Delmas, F.; Keita, A.; Doumbo, O.; Balansard, G.; Timon-David, P. Antimalarial activity of four plants used in traditional medicine in Mali. Phytother. Res. 2000, 14, 45–47. [Google Scholar] [CrossRef]
- Duker-Eshun, G.; Jaroszewski, J.W.; Asomaning, W.A.; Oppong-Boachie, F.; Brogger Christensen, S. Antiplasmodial constituents of Cajanus cajan. Phytother. Res. 2004, 18, 128–130. [Google Scholar] [CrossRef]
- Barea, C.; Pabón, A.; Galiano, S.; Pérez-Silanes, S.; Gonzalez, G.; Deyssard, C.; Monge, A.; Deharo, E.; Aldana, I. Antiplasmodial and leishmanicidal activities of 2-cyano-3-(4-phenylpiperazine-1-carboxamido) quinoxaline 1,4-dioxide derivatives. Molecules 2012, 17, 9451–9461. [Google Scholar] [CrossRef]
- Thibeault, D.; Gauthier, C.; Legault, J.; Bouchard, J.; Gagné, L.; Pichette, A. Synthesis and cytotoxicity of lupane-type triterpenoid glyceryl esters. Bioorg. Med. Chem. Lett. 2012, 22, 4735–4739. [Google Scholar] [CrossRef]
- Urban, M.; Vlk, M.; Dzubak, P.; Hajduch, M.; Sarek, J. Cytotoxic heterocyclic triterpenoids derived from betulin and betulinic acid. Bioorg. Med. Chem. Lett. 2012, 20, 3666–3674. [Google Scholar] [CrossRef]
- Gazarini, M.L.; Thomas, A.P.; Pozzan, T.; Garcia, C.R.S. Calcium signaling in a low calcium environment: How the intracellular malaria parasite solves the problem. Cell Biol. 2003, 161, 103–110. [Google Scholar] [CrossRef]
- Varotti, F.P.; Beraldo, F.H.; Gazarini, M.L.; Garcia, C.R.S. Plasmodium falciparum malaria parasites display a THG-sensitive Ca2+ pool. Cell Calcium 2003, 33, 137–144. [Google Scholar] [CrossRef]
- Trager, W.; Jensen, J.B. Human malaria parasites in continuous culture. Science 1976, 193, 673–675. [Google Scholar]
- Lambros, C.; Vanderberg, J.P.J. Synchronization of Plasmodium falciparum erythrocytic stages in culture. Parasitology 1979, 65, 418–420. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Innocente, A.M.; Silva, G.N.S.; Cruz, L.N.; Moraes, M.S.; Nakabashi, M.; Sonnet, P.; Gosmann, G.; Garcia, C.R.S.; Gnoatto, S.C.B. Synthesis and Antiplasmodial Activity of Betulinic Acid and Ursolic Acid Analogues. Molecules 2012, 17, 12003-12014. https://doi.org/10.3390/molecules171012003
Innocente AM, Silva GNS, Cruz LN, Moraes MS, Nakabashi M, Sonnet P, Gosmann G, Garcia CRS, Gnoatto SCB. Synthesis and Antiplasmodial Activity of Betulinic Acid and Ursolic Acid Analogues. Molecules. 2012; 17(10):12003-12014. https://doi.org/10.3390/molecules171012003
Chicago/Turabian StyleInnocente, Adrine M., Gloria N. S. Silva, Laura Nogueira Cruz, Miriam S. Moraes, Myna Nakabashi, Pascal Sonnet, Grace Gosmann, Célia R. S. Garcia, and Simone C. B. Gnoatto. 2012. "Synthesis and Antiplasmodial Activity of Betulinic Acid and Ursolic Acid Analogues" Molecules 17, no. 10: 12003-12014. https://doi.org/10.3390/molecules171012003





