1. Introduction
The highly extended geographic distribution of French Polynesia in the Pacific Ocean has resulted in a relatively high ratio of endemic species spread over the plethora of islands constitutive of this territory, whose distance from the continents may favor the “insular syndrome” leading to more speciation processes [
1]. The Marquesas archipelago is one of the most isolated archipelagos of French Polynesia, which have a very original flora within a high endemicity [
2,
3]. Among them, the plant
Rauvolfia nukuhivensis (Apocynaceae), locally called “tueiao”, is endemic of the Marquesas archipelago and more precisely of the small island of Nuku Hiva. Although this plant is still used in traditional medicine as a gynecological antiseptic [
4], no phytochemical study has been reported so far. Over-exploited because of the frequent use of its bark (macerate) by local communities, the plant is now classified as a critically endangered species, and the description of its chemical constituents is urgently needed before its extinction [
5]. Plants of the genus
Rauvolfia are well known for their prolific biosynthesis of structurally diverse bioactive indole alkaloids, among them ajmaline and its plethora of structural analogues [
6]. Nowadays, the biosynthetic genes of these biologically important alkaloids have been identified, which is an important step towards a commercial use of these compounds [
7,
8,
9]. Some phytochemical work has been previously undertaken on Pacific species of the genus
Rauvolfia. The Scheuer group identified serpentinine, ajmaline, sandwicine, sandwicencine, tetraphylline, tetraphyllicine and mauiensine from the Hawaiian spp.
Rauvolfia sandwicensis and
R. mauiensis [
10,
11]. Later, the Sevenet group identified ajmaline, but not reserpine, in some endemic species of the genus
Rauvolfia from New Caledonia [
12].
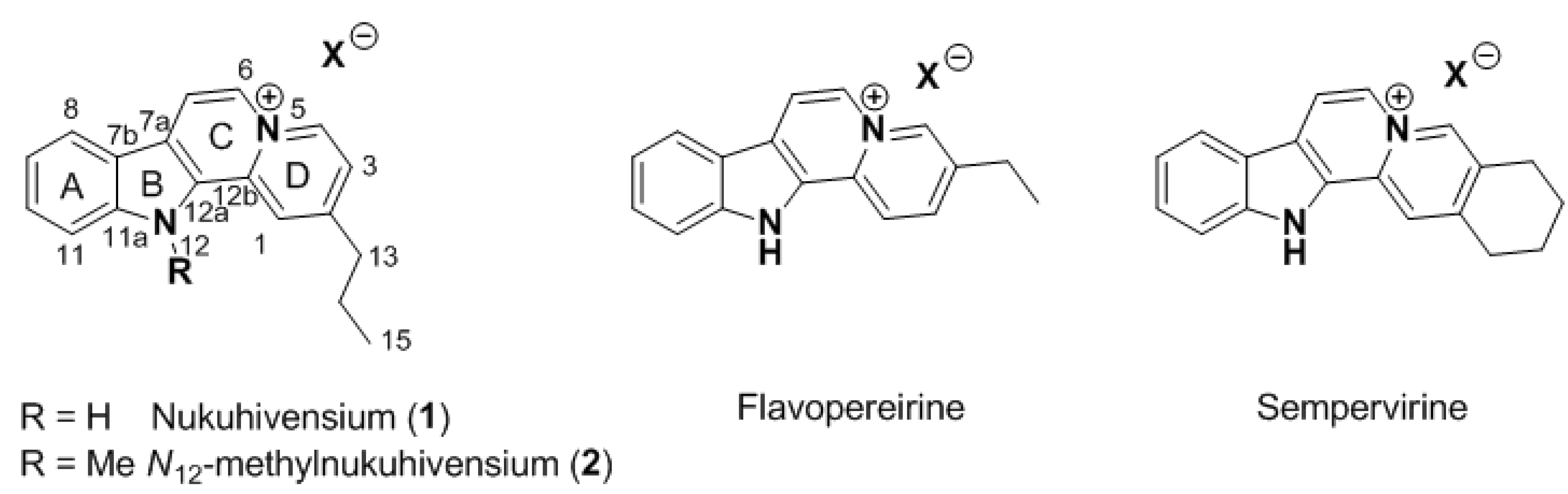
Within the framework of our continued interest in the phytochemical study of endemic and endangered species of French Polynesia, two original indolo[2,3-
a]quinolizinium derivatives named nukuhivensium (
1) and
N12-methylnukuhivensium (
2) were isolated from the Polynesian sp.
Rauvolfia nukuhivensis (
Figure 1). We report herein the isolation and structure elucidation of these new derivatives, as well as their antimicrobial activities.
Figure 1.
Structures of natural indolo[2,3-a]quinoliziniums.
Figure 1.
Structures of natural indolo[2,3-a]quinoliziniums.
2. Results and Discussion
The stem bark of Rauvolfia nukuhivensis was extracted three times with ethanol and the crude extract was submitted to two subsequent fractionation processes, first by reversed phase and then by normal phase chromatography, to yield two main fractions containing alkaloids as revealed by TLC. Purification of one fraction by reversed phase HPLC led to the isolation of both compounds 1 and 2 in their pure form.
The molecular formula of
1 was determined as C
18H
17N
2+ by HRESIMS. The
1H-NMR data (
Table 1) suggested the presence of a polyaromatic system substituted by an
n-propyl group corresponding to the signals at
δH 1.12 (t,
3J = 7.5 Hz, H
3-15), 1.94 (tq,
3J = 7.7 and 7.5 Hz, H
2-14) and 3.06 (t,
3J = 7.7 Hz, H
2-13) ppm which were clearly COSY correlated. Inspection of the chemical shifts of the resulting protons and carbons indicated that the rest of the molecule was polyaromatic. The presence of heterocyclic aromatic systems was deduced from the high deshielding of some resonating protons.
Table 1.
1H (500 MHz) and 13C (125 MHz) NMR data for compounds 1 and 2 in CD3OD.
Table 1.
1H (500 MHz) and 13C (125 MHz) NMR data for compounds 1 and 2 in CD3OD.
| Position | 1 | 2 |
|---|
| δH, mult (J in Hz) | δC, mult | HMBC (H→C) | δH, mult (J in Hz) | δC, mult |
|---|
| 1 | 8.65, s | 120.5, CH | 13, 3, 6, 12a, 12b | 8.96, s | 121.5, CH |
| 2 | | 154.4, C | | | 154.1, C |
| 3 | 7.83, d (7.0) | 124.4, CH | 4, 1, 13 | 7.86, d (7.0) | 123.9, CH |
| 4 | 9.19, d (7.0) | 137.6, CH | 3, 6, 2, 12b | 9.24, d (7.0) | 138.4, CH |
| 6 | 8.84, d (7.0) | 128.1, CH | 7, 7a, 12b, 4 | 8.90, d (7.0) | 128.1, CH |
| 7 | 8.61, d (7.0) | 117.2, CH | 7b, 6, 12a, 12b | 8.66, d (7.0) | 116.8, CH |
| 7a | | 125.0, C | | | 125.6, C |
| 7b | | 122.5, C | | | 128.9, C |
| 8 | 8.36, d (8.0) | 123.3, CH | 11, 7b, 7a | 8.41, d (8.0) | 122.8, CH |
| 9 | 7.50, dd (8.0; 7.0) | 122.9, CH | 11, 8, 10, 11a, 7b | 7.54, dd (8.0, 7.0) | 123.5, CH |
| 10 | 7.74, dd (8.0; 7.0) | 131.0, CH | 8, 7b | 7.83, dd (8.0, 7.0) | 131.2, CH |
| 11 | 7.84, d (8.0) | 113.6, CH | 9, 7b, 11a | 7.95, d (8.0) | 112.1, CH |
| 11a | | 143.3, C | | | 144.9, C |
| 12 | | | | 4.58, s | 34.6, CH3 |
| 12a | | 131.7, C | | | 131.4, C |
| 12b | | 134.3, C | | | 135.2, C |
| 13 | 3.06, t (7.7) | 38.6, CH2 | 14, 15, 2, 1, 3 | 3.09, t (7.7) | 38.7, CH2 |
| 14 | 1.94, tq (7.7; 7.5) | 23.9, CH2 | 15, 13, 2 | 1.93, tq (7.7, 7.5) | 24.4, CH2 |
| 15 | 1.12, t (7.5) | 14.0, CH3 | 14, 13 | 1.12, t (7.5) | 14.0, CH3 |
A careful inspection of the HMBC spectrum allowed us to unravel the chemical structure of
1. Indeed, the
n-propyl was located on the
para position of a pyridinium ring due to the key H
2-13/C-2/C-1/C-3 HMBC correlations, the H
2-13/H-1/H-3
4J correlations and the
3J COSY correlation between the signal at
δH 7.83 (d,
3J = 7.0 Hz, H-3) and 9.19 (d,
3J = 7.0 Hz, H-4) ppm, this highly deshielded signal being inferred to the vicinity of the electron withdrawing effect of an ammonium group (
Figure 2). The clear H-4/C-3/C-2/C-6/C-12b
2J and
3J HMBC correlations allowed the assignment of the entire pyridinium D ring as well as the C-6 methine. Starting from this C-6 methine, COSY coupled to the signal at
δH 8.61 (d,
3J = 7.0 Hz, H-7), we were able to build the quinolizinium part of the molecule. Indeed the H-6/C-4/C-12b/C-7/C-7a HMBC correlations yielded the construction of the second pyridinium ring. The pattern of the resulting signals in the
1H-NMR and COSY spectra was reminiscent of an indole ring with
δH 8.36 (d,
3J = 8.0 Hz, H-8), 7.50 (dd,
3J = 8.0 and 7.0 Hz, H-9), 7.74 (dd,
3J = 8.0 and 7.0 Hz, H-10) and 7.84 (d,
3J = 8.0 Hz, H-11) ppm. Finally, the indole ring was fused to the second pyridinium at C-12a/C-7a due to the key H-7/C-7b/C-12a/C-7a HMBC correlations. Additional H-8/C-7a/C-11a
3J HMBC correlations came to confirm the proposed structure of
1.
Figure 2.
Key COSY (bold) and HMBC (H → C, arrow) correlations for 1.
Figure 2.
Key COSY (bold) and HMBC (H → C, arrow) correlations for 1.
The molecular formula of
2 was determined as C
19H
19N
2+ by HRESIMS and suggested the presence of an additional methyl group comparing to
1. This methyl group was easily located at
N12 due to the chemical shifts at
δH 4.58 (s,
H3-C) and
δC 33.6 (
C-H
3) ppm (
Table 1) which were reminiscent of an aromatic
N-substituted methyl group. The deshielding of H-1 was clearly induced by the presence of this methyl at
N12 and the
H3-C/C-11a/C-12a HMBC correlations finally confirmed the substitution at
N12. Compound 2 may be seen as an artifact during our extraction and purification process. Nevertheless, methylation with methanol is quite rare, especially under our mild extraction and purification conditions and the low nucleophilicity of the indole nitrogen.
From a structural point of view, these compounds share a rare indolo[2,3-
a]quinolizinium core already found in flavopereirine [
13,
14] and sempervirine [
15], for example (
Figure 1). To our knowledge, the presence of an alkyl chain at C-2 was never reported on this aromatic core and this observation raised the question of the biosynthesis of these compounds. While for flavopereirine and sempervirine an oxidative process starting from saturated known analogues can be proposed, the presence of an
n-propyl moiety at C-2 was very intriguing. Because there was no doubt on the structure of these compounds, we undertook a detailed analysis of known biochemical pathways leading to these new compounds. We suggest that a plausible hypothesis would start from the key and highly reactive intermediate “dialdehyde” derived from strictosidine (
Scheme 1) [
16]. In other
Rauvolfia species, this dialdehyde has been proposed to yield the sarpagan and ajmalan alkaloids via 4,21-dehydro-geissoschizine, which involves an
iso-propyl at C-2. Rather than an unlikely rearrangement of these three carbon atoms, we propose that nukuhivensiums could be formed like vallesiachotamine (
Scheme 1). Indeed, after an alternative cyclization of “dialdehyde” two subsequent decarboxylative steps could afford the
n-propyl at C-2 and finally compound
1 after additional oxidative steps. Work is ongoing to isolate minor alkaloids from this plant in order to strengthen our biosynthetic hypothesis.
Scheme 1.
Biosynthetic hypothesis for 1.
Scheme 1.
Biosynthetic hypothesis for 1.
Because extracts of this plant are being used as a gynecological antiseptic, we first decided to assess the antimicrobial potential of these two new derivatives. Compounds
1 and
2 were evaluated
in vitro for their antimicrobial activity against
Escherichia coli,
Staphylococcus aureus,
Candida albicans and
Aspergillus niger. Both compounds exhibited low antimicrobial activities against
S. aureus and
C. albicans (
Table 2). Two options can then be proposed: (i) other minor compounds are responsible of an antimicrobial activty related to the ethnopharmocological use of this plant; or (ii) the mode of action related to the gynecological use of this plant is not as antimicrobial agents.
Table 2.
Antimicrobial activities of 1 and 2.
Table 2.
Antimicrobial activities of 1 and 2.
| Compounds | MIC90 S. aureus (µg/mL) | MIC90 E. coli (µg/mL) | MIC90 C. albicans (µg/mL) | MIC90 A. niger (µg/mL) |
|---|
| 1 | 105 | >150 | 100 | >150 |
| 2 | 115 | >150 | 100 | >150 |
| Tetracycline | 0.5 | 3 | Nt | Nt |
| Econazole | Nt | Nt | 2.60 | 2.20 |
3. Experimental
3.1. General
UV-Vis spectra were recorded by HPLC-DAD. NMR spectra were measured on a Bruker Avance 500 MHz spectrometer with pulsed field gradient and signals were referenced to the residual solvent signals (CD3OD, at δH 3.31 and δC 49.0 ppm). HRESIMS data were measured with a LTQ Orbitrap mass spectrometer (Thermo Finnigan). HPLC purification was carried out on a Waters 600 system equipped with a Waters 717 Plus autosampler, a Waters 998 photodiode array detector, and a Sedex 75 evaporative light-scattering detector (Sedere, France).
3.2. Plant Material
Rauvolfia nukuhivensis was collected at Maauu in the “Terre Déserte” area on Nuku Hiva Island, Marquesas archipelago, French Polynesia, at 477 m above sea level and identified by Dr Jean-François Butaud. A voucher specimen (JFB 2808) has been deposited in the Herbarium of French Polynesia.
3.3. Extraction and Isolation
The dried bark (250 g) was ground and extracted three times with 750 mL of EtOH at room temperature. The maceration was allowed to proceed for 16 h and then the solvent was filtered and concentrated by evaporation to yield a crude oil. The resulting extract (21 g) was dissolved by sonication in a mixture of 200 mL MeOH/CH2Cl2 (1:1) and then filtered before fractionation. This was carried out by vacuum liquid chromatography on RP-C18 and eluted with solvents of decreasing polarity A, B, C, D, E (H2O, H2O/MeOH (1:1), MeOH, MeOH/CH2Cl2 (1:1) and CH2Cl2, respectively). Fraction D, obtained with the MeOH/CH2Cl2 eluent, provided 2.82 g of an oily residue. A portion of fraction D (1.41 g) was further fractionated by normal phase (diol) flash column chromatography using solvents of stepwise increasing polarity: cyclohexane, cyclohexane/EtOAc (3:1), cyclohexane/EtOAc (1:1), cyclohexane/EtOAc (1:3), EtOAc, EtOAc/MeOH (3:1), EtOAc/MeOH (1:1), EtOAc/MeOH (1:3) and MeOH, yielding nine fractions D1 to D9. Purification of D7 (104 mg) was performed by RP-C18 semi-preparative HPLC (Phenomenex, Luna 5 µm C18, 250 mm × 10 mm) with a gradient of MeOH/H2O/TFA (from 70:30:0.1 to 30:70:0.1, flow 3 mL min−1) to afford pure compounds 1 (4.7 mg) and 2 (2.8 mg).
Nukuhivensium (
1). UV measured by HPLC/DAD (MeOH/H
2O/TFA)
λmax 224, 241, 290, 340, 382 nm;
1H- and
13C-NMR see
Table 1; HRESIMS
m/z 261.13953 [M]
+ (calcd for C
18H
17N
2+, 261.13917).
N
12-methylnukuhivensium (2). UV measured by HPLC/DAD (MeOH/H
2O/TFA)
λmax 225, 237, 248, 287, 335, 393 nm;
1H- and
13C-NMR see
Table 1; HRESIMS
m/z 275.15417 [M]
+ (calcd for C
19H
19N
2+, 275.15482).
3.4. Biological Evaluations
Reference strains of Escherichia coli (ATCC 8739) and Staphylococcus aureus subsp. aureus (ATCC 6538) were obtained from the Collection of the Institute Pasteur (Paris, France). Bacterial species were cultivated for 24 h in Luria Bertani medium (LB) at 37 °C. Candida albicans (IBMC Strasbourg) and Aspergillus niger (ATCC 9142) were cultivated at 30 °C for 48 h in Sabouraud dextrose medium (Sanofi Diagnostic Pasteur). Briefly, pre-cultures of the tested micro-organisms were made by inoculating 10 mL of LB and incubating for 24 h for bacteria or 10 mL in Sabouraud for 48 h for fungi. A culture suspension were made by 1/1,000 dilution from preculture and seeded in 96-well microtitration plates. Four microliters of two-fold serial dilutions of each compound (10 mg/mL) was prepared in 100 μL of medium. The plates were incubated at 37 °C for bacteria and at 30 °C for fungus. After 24 h, the optical density of the bacterial suspension of each well was measured at 595 nm using a multiplate reader. Tetracycline and econazole were used as positive controls.