Synthesis and Field Evaluation of the Sex Pheromone Analogues to Soybean Pod Borer Leguminivora glycinivorella
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
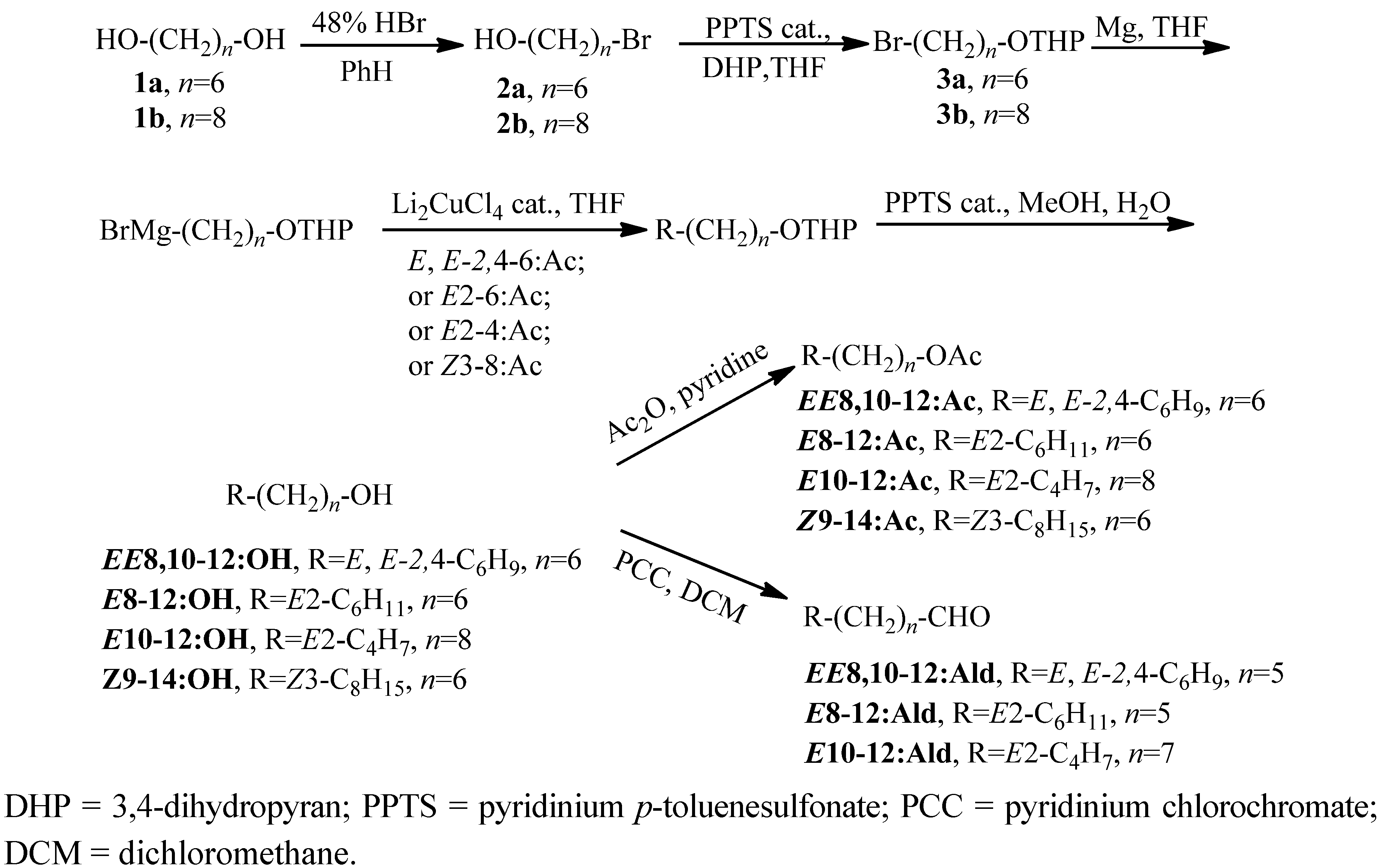
2.2. Field Tests
| Lure components | Dose (mg/lure) | No. of males captured/trap/day a |
|---|---|---|
| 0.01 | 1.42 ± 0.22 defg | |
| 12:Ac | 0.1 | 1.29 ± 0.18 defg |
| 0.5 | 1.58 ± 0.33 cdefg | |
| 0.01 | 1.75 ± 0.21 cdefg | |
| 14:Ac | 0.1 | 3.45 ± 0.47 c |
| 0.5 | 0.80 ± 0.41 efg | |
| 0.01 | 0.33 ± 0.17 g | |
| EE-8,10-12:OH | 0.1 | 0.58 ± 0.08 g |
| 0.5 | 0.29 ± 0.04 g | |
| 0.01 | 0.83 ± 0.18 efg | |
| EE-8,10-12:Ac | 0.1 | 10.56 ± 1.41 a |
| 0.5 | 1.17 ± 0.15 defg | |
| 0.01 | 7.33 ± 0.86 b | |
| E-8-12:OH | 0.1 | 2.75 ± 0.21 cde |
| 0.5 | 0.74 ± 0.26 efg | |
| 0.01 | 0.29 ± 0.08 g | |
| E-8-12:Ac | 0.1 | 0.29 ± 0.11 g |
| 0.5 | 0.33 ± 0.04 g | |
| 0.01 | 0.67 ± 0.22 fg | |
| E-10-12:OH | 0.1 | 0.38 ± 0.12 g |
| 0.5 | 0.58 ± 0.18 g | |
| 0.01 | 0.54 ± 0.04 g | |
| E-10-12:Ac | 0.1 | 10.25 ± 0.54 a |
| 0.5 | 6.58 ± 0.60 b | |
| 0.01 | 1.19 ± 0.09 defg | |
| EE-8,10-12:Ald | 0.1 | 0.62 ± 0.19 fg |
| 0.5 | 1.14 ± 0.18 defg | |
| 0.01 | 0.92 ± 0.15 defg | |
| E-8-12:Ald | 0.1 | 0.50 ± 0.07 g |
| 0.5 | 1.58 ± 0.18 cdefg | |
| 0.01 | 0.84 ± 0.10 efg | |
| E-10-12:Ald | 0.1 | 1.04 ±0.11 defg |
| 0.5 | 0.88 ± 0.26 defg | |
| 0.01 | 0.62 ± 0.21 fg | |
| Z-9-14:OH | 0.1 | 0.88 ± 0.14 defg |
| 0.5 | 0.87 ± 0.33 defg | |
| 0.01 | 1.08 ± 0.36 defg | |
| Z-9-14:Ac | 0.1 | 2.62 ± 0.14 cdef |
| 0.5 | 2.87 ± 0.44 cd | |
| Control (blank) | 0 | 0.08 ± 0.04 g |
| Control (solvent) | 0 | 0.15 ± 0.07 g |
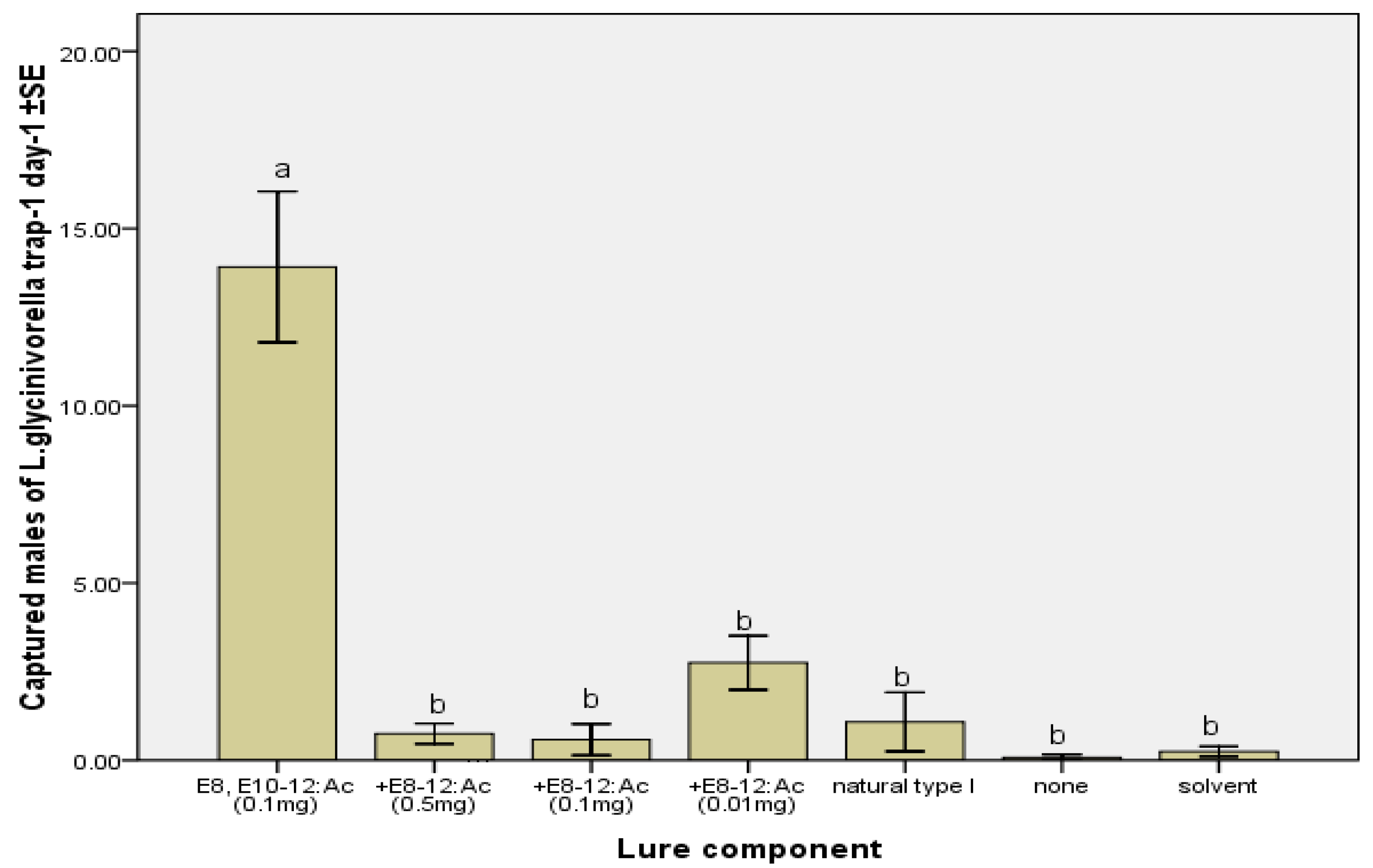

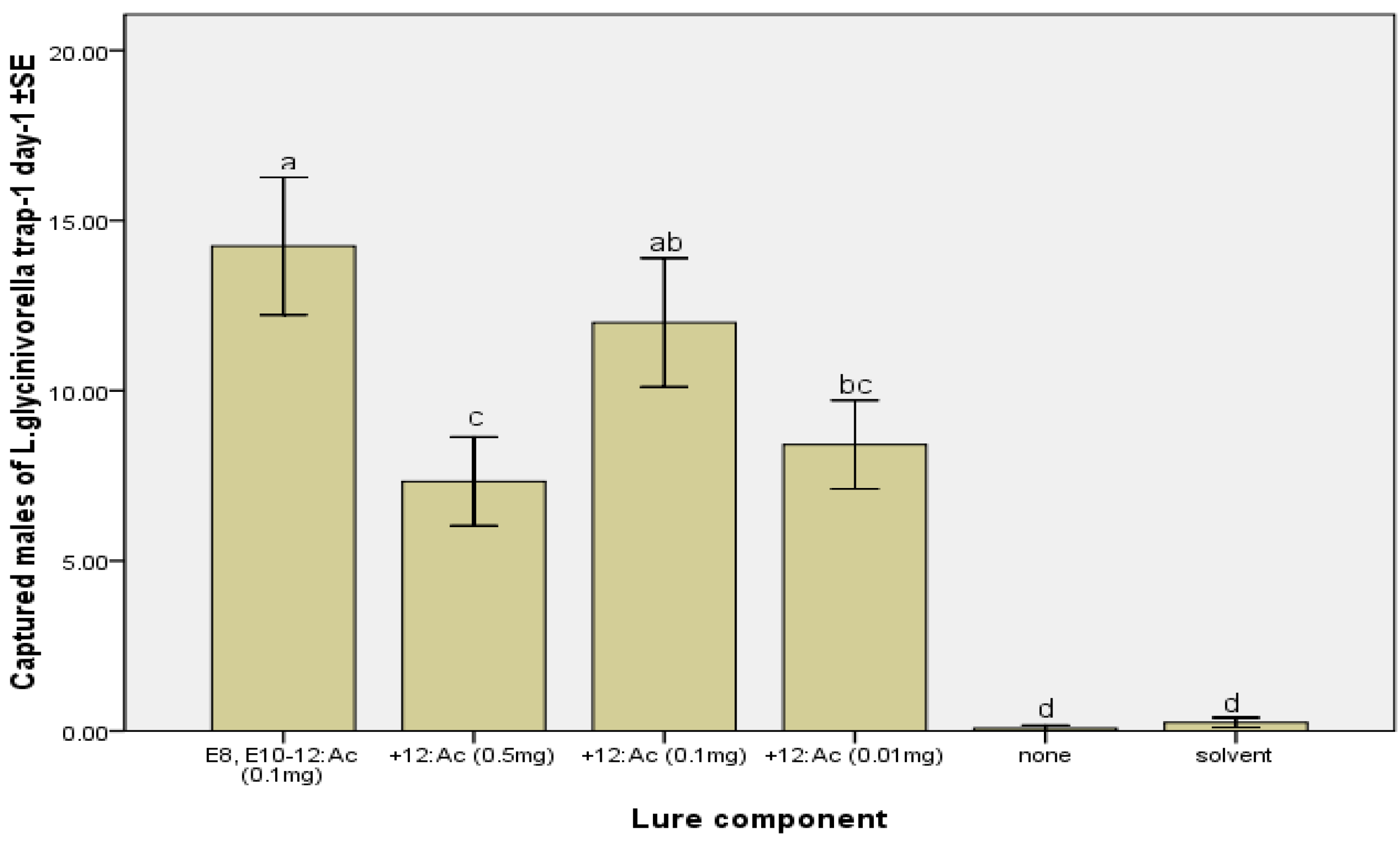
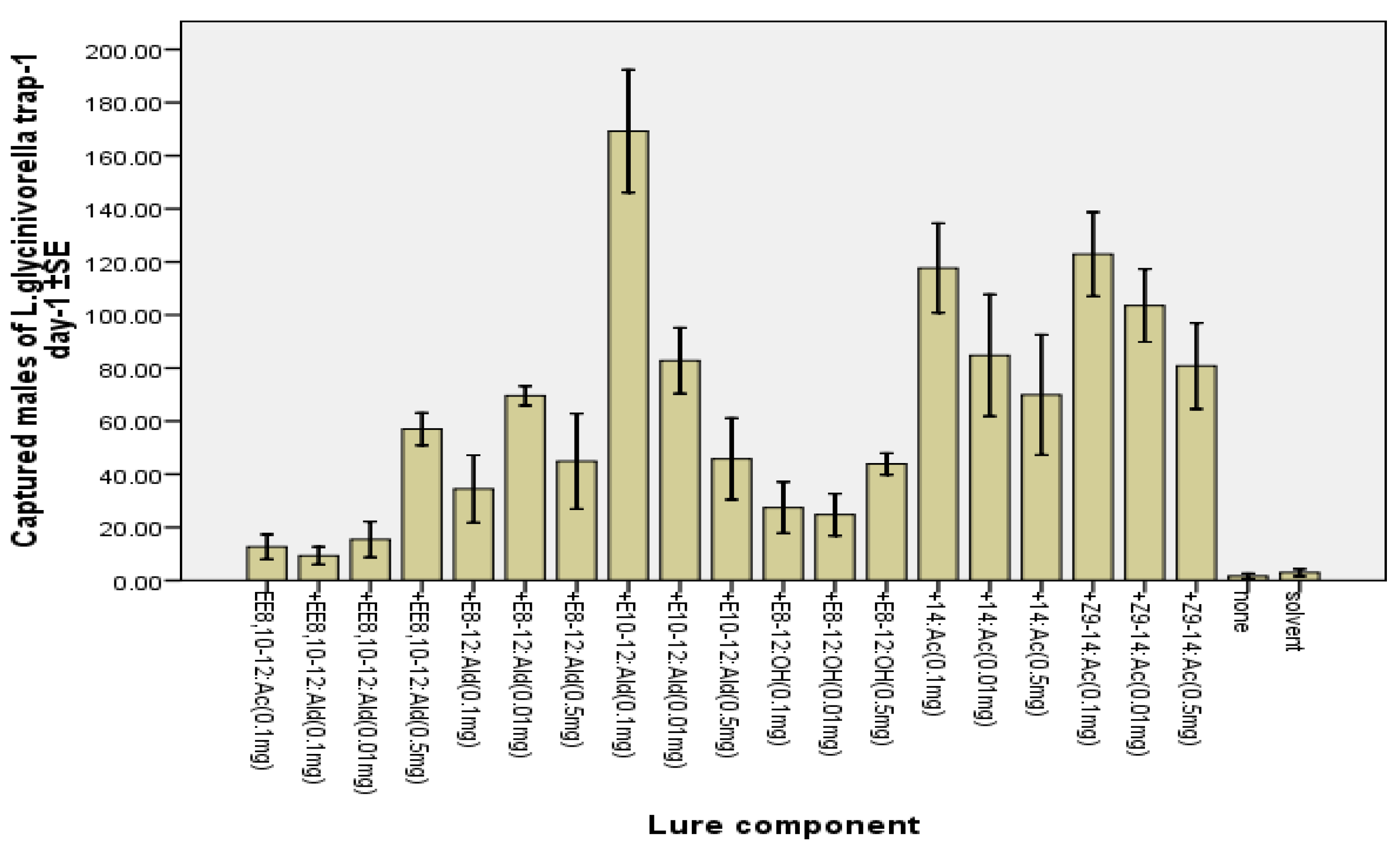
3. Experimental
3.1. General
3.2. Field Trapping Experiments
3.3. Statistical Analysis
4. Conclusions
Acknowledgements
- Sample Availability: Samples of the compounds EE-8,10-12:OH, EE-8,10-12:Ac, E-8-12:OH, E-8-12:Ac, E-10-12:OH, E-10-12:Ac, EE-8,10-12:Ald, E-8-12:Ald, E-10-12:Ald, Z-9-14:OH, and Z-9-14:Ac are available from the authors.
References
- Vang, L.V.; Ishitani, M.; Komai, F.; Yamamoto, M.; Ando, T. Sex pheromone of the soybean pod borer, Leguminivora. glycinivorella (Lepidoptera:Tortricidae): Identification and field evaluation. Appl. Entomol. Zool. 2006, 41, 507–513. [Google Scholar] [CrossRef]
- Wu, C.Y.; Hsien, M.F.; Yen, Y.P. Facile synthesis of the sex pheromone components of the legume pod borer and soybean pod borer by Suzuki-Miyaura cross-coupling reaction. Lett. Org. Chem. 2008, 5, 514–517. [Google Scholar] [CrossRef]
- Wang, C.Y.; Liu, J.; Song, F.R.; Xin, B.M.; Guo, S.G.; Zhou, Z.P.; Xu, J.W.; Wang, Z.H. The chemical structure, EAG and field trapping effect of soybean podborer sex pheromone. Acta Phytophylacica Sin. 1992, 19, 331–335. [Google Scholar]
- Marques, F.D.A.; McElfresh, J.S.; Millar, J.G. Kovats retention indexes of monounsaturated C12, C14, and C16 alcohols, Acetates and aldehydes commonly found in lepidopteran pheromone blends. J. Braz. Chem. Soc. 2000, 11, 592–599. [Google Scholar] [CrossRef]
- Witzgall, P.; Bengtsson, M.; Rauscher, S.; Liblikas, I.; Backman, A.C.; Coracini, M. Identification of further sex pheromone synergists in the codling moth, Cydia. Pomonella. Entomol. Exp. Appl. 2001, 101, 131–141. [Google Scholar]
- Mori, K. Simple synthesis of sex pheromones of codling moth and red bollworm moth by the coupling of Grignard reagents with allylic halides. Tetrahedron 1974, 30, 3807–3810. [Google Scholar] [CrossRef]
- Samain, D.; Descoins, C. A short, Stereoselective synthesis of 8E, 10E-dodecadien-1-ol; the sex pheromone of the codling moth, Laspeyresia. pomonella, L. Synthesis 1978, 1978, 388–389. [Google Scholar] [CrossRef]
- Wakamura, S. Identification of sex pheromone components of the podborer, Matsumuraesed. falcana (Walshingham [sic]) (Lepidoptera: Tortricidae). Entomol. Exp. Appl. 1985, 20, 189–198. [Google Scholar]
- Wakamura, S.; Hattori, M.; Igita, K.; Yasuda, K. Tridjaka, Sex pheromone of Etiella. behrii, A pod borer of soybean in Indonesia: Identification and field attraction. Entomol. Exp. Appl. 1999, 91, 413–420. [Google Scholar]
- Tóth, M.; Löfstedt, C.; Hansson, B.S.; Szöcs, G.; Farag, A.I. Identification of four components from the female sex pheromone of the lima-bean pod borer, Etiella. zinckenella . Entomol. Exp. Appl. 1989, 51, 107–112. [Google Scholar] [CrossRef]
- Tóth, M.; Talekar, N.S.; Szócs, G. Optimization of blends of synthetic pheromone components for trapping male limabean pod bores (Etiella. zinckenella Tr.) (Lepidoptera: Phycitidae): Preliminary evidence on geographical differences. Bioorg. Med. Chem. 1996, 4, 495–497. [Google Scholar] [CrossRef]
- Tabata, J.; Yokosuka, T.; Hattori, M.; Ohashii, M.; Noguchi, H.; Sugie, H. Sex attraction pheromone of the limabean pod borer, Etiella. zinckenella (Treitschke) (Lepidoptera: Pyralidae), in Japan. Entomol. Exp. Appl. 2008, 43, 351–358. [Google Scholar]
- Li, X.A.; Wang, Q.B.; He, M.Y.; Yuan, G. Highly stereoselective synthesis and identification of (9E, 11E)-tetradecadienyl acetate (insect sex pheromone of Epiphyas. Postvittana.). Chemistry 2002, 3, 179–181. [Google Scholar]
- Yan, R.A.; He, Z.Y.; Su, J.Y.; Zeng, L.M. Preparation of PCC and its application in organic synthesis. Chem. Reag. 2000, 22, 305–306. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hu, D.-H.; He, J.; Zhou, Y.-W.; Feng, J.-T.; Zhang, X. Synthesis and Field Evaluation of the Sex Pheromone Analogues to Soybean Pod Borer Leguminivora glycinivorella. Molecules 2012, 17, 12140-12150. https://doi.org/10.3390/molecules171012140
Hu D-H, He J, Zhou Y-W, Feng J-T, Zhang X. Synthesis and Field Evaluation of the Sex Pheromone Analogues to Soybean Pod Borer Leguminivora glycinivorella. Molecules. 2012; 17(10):12140-12150. https://doi.org/10.3390/molecules171012140
Chicago/Turabian StyleHu, Dai-Hua, Jun He, Yi-Wan Zhou, Jun-Tao Feng, and Xing Zhang. 2012. "Synthesis and Field Evaluation of the Sex Pheromone Analogues to Soybean Pod Borer Leguminivora glycinivorella" Molecules 17, no. 10: 12140-12150. https://doi.org/10.3390/molecules171012140




