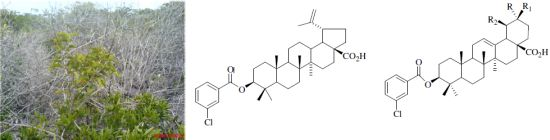
Bioactive Oleanane, Lupane and Ursane Triterpene Acid Derivatives
Abstract
:1. Introduction
2. Results and Discussion
| Compound | Solvent | δ H-3 |
|---|---|---|
| 1 | Py | 3.5 ( t, 7.0 Hz) |
| 1a | Cd | 4.48 ( t, 7.7 Hz) |
| 1b | Cd | 4.48 ( t, 7.7 Hz) |
| 1c | Ac | 3.9 ( t, 7.7 Hz) |
| 1d | Ac | 3.7 ( t, 7.1 Hz) |
| 2 | Py | 3.5 ( t, 8.0 Hz) |
| 2a | Cd | 4.51 ( t, 7.7 Hz) |
| 2b | Ac | 4.49 ( m, 7.7 Hz) |
| 2c | Ac | 4.7 ( t, 7.7 Hz) |
| 2d | Ac | 4.35 ( t, 7.7 Hz) |
| 3 | Py | 3.48 ( t, 7.8 Hz) |
| 3a | Cd | 4.51 ( dd, 6.0 and 8.6 Hz) |
| 3b | Cd | 4.51 ( dd, 5.6 e 8.9 Hz) |
| 3c | Cd | 4.76 ( dd, 5.6 e 8.9 Hz) |
| 3d | Cd | 4.75 ( t, 8.9 Hz) |
| Compound | Lethality towards Artemia salina | |
|---|---|---|
| CL50 (µg/mL) | SD | |
| 1a | >1,000 μg/mL | - |
| 1b | >1,000 μg/mL | - |
| 1c | >1000 μg/mL | - |
| 1d | 117.1 μg/mL | 0.418 |
| 2a | >1,000 μg/mL | |
| 2b | >1,000 μg/mL | - |
| 2c | >1,000 μg/mL | - |
| 2d | 477.2 μg/mL | 0.304 |
| 3a | >1,000 μg/mL | - |
| 3b | >1,000 μg/mL | - |
| 3c | >1,000 μg/mL | - |
| 3d | >1,000 μg/mL | - |
| Compound | IC50 ± RSD (μg/mL) |
|---|---|
| 2d | 1444 ± 2.0 |
| 1d | 23.41 ± 0.9 |
| 2a | 44.58 ± 0.7 |
| Quercetin (positive control) | 23.18 ± 1.4 |
3. Experimental
3.1. General Procedures
3.2. Plant Material
3.3. Extraction and Isolation
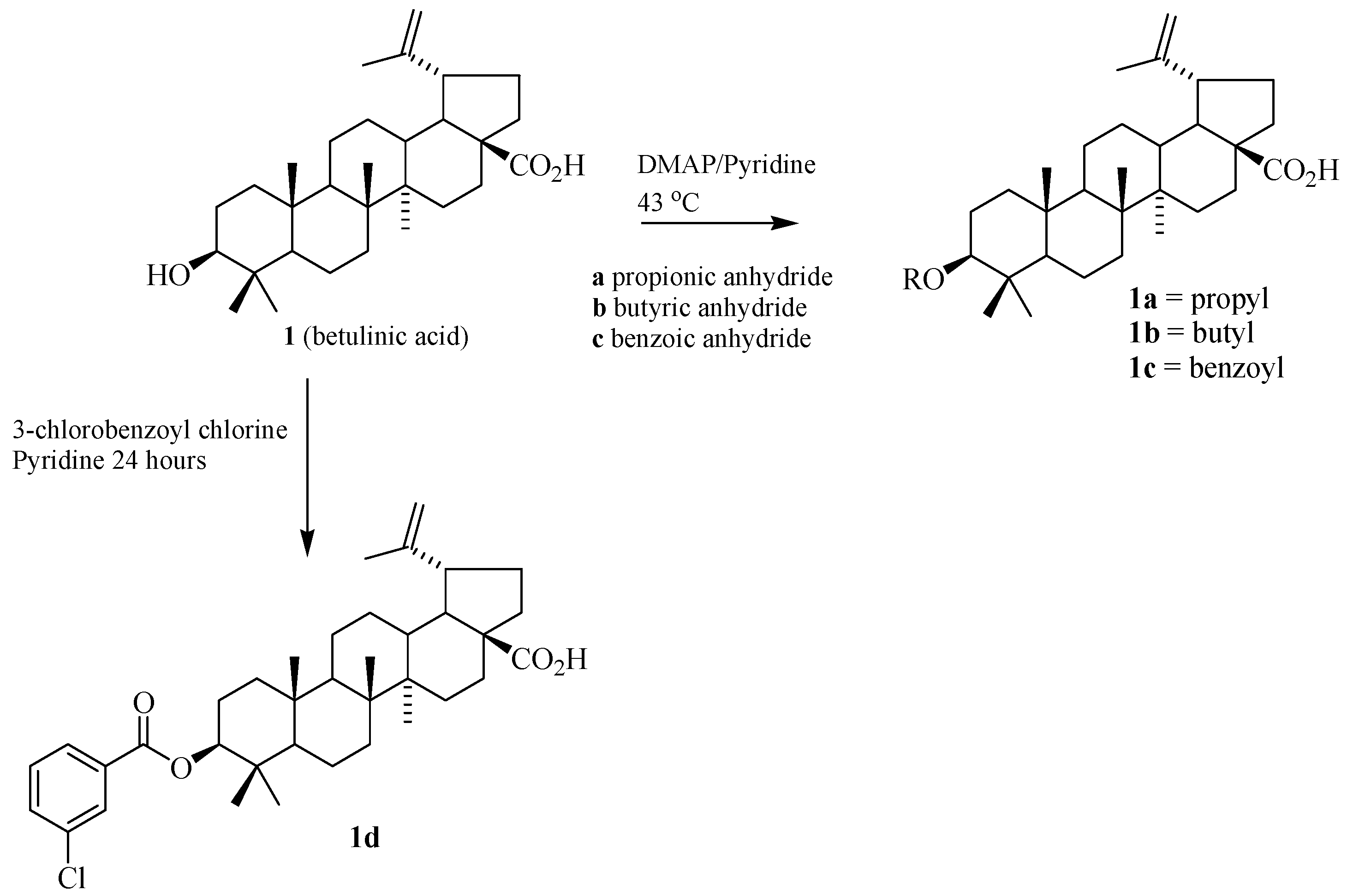
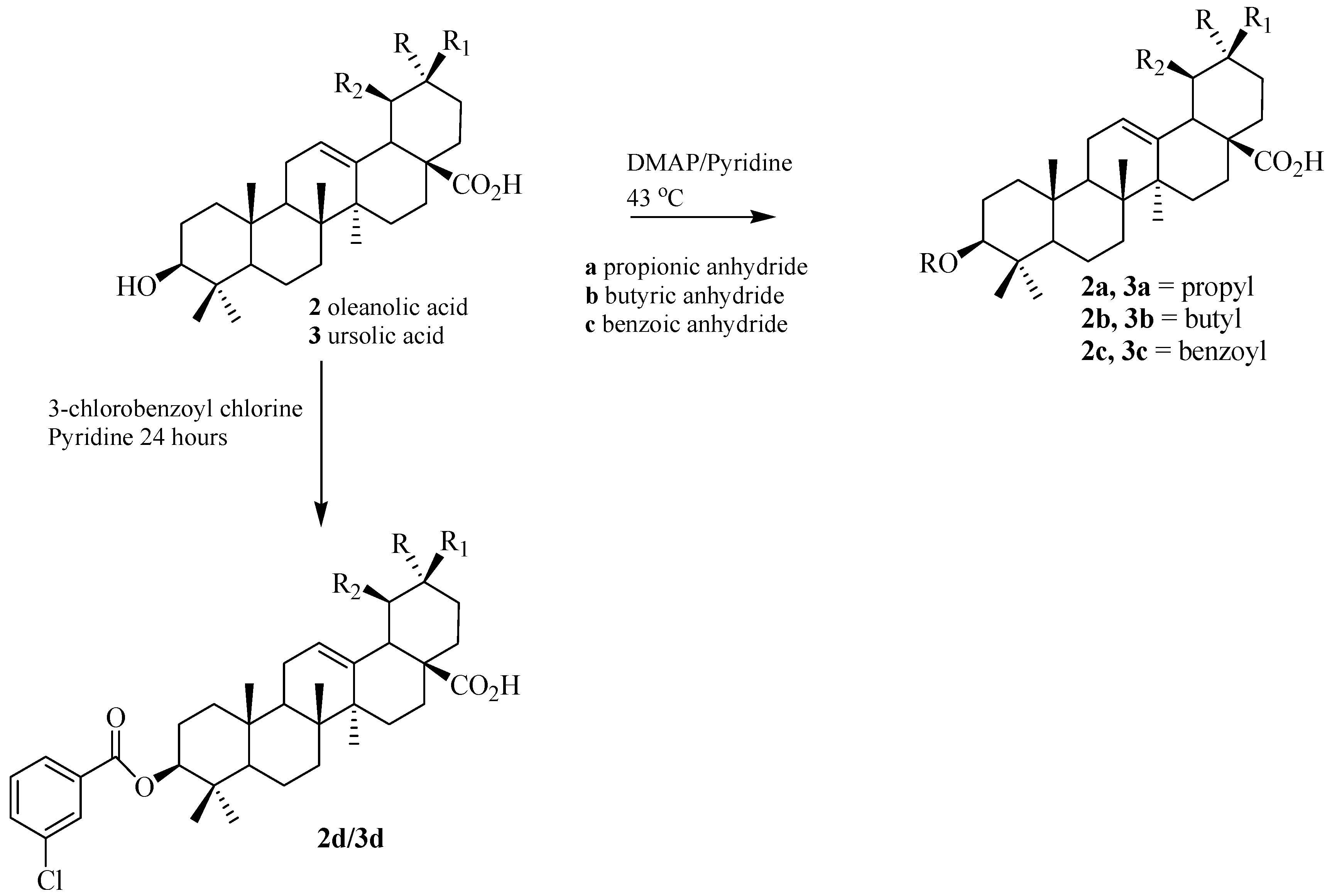
3.4. Synthesis of the Ester Derivatives
| Triterpene acid derivatives | m (mg) | Yield (%) |
|---|---|---|
| 1a | 1.7 | 10 |
| 1b | 1.7 | 10 |
| 1c | 13.5 | 56 |
| 1d | 8.1 | 33 |
| 2a | 1.4 | 9 |
| 2b | 1.3 | 7 |
| 2c | 8.1 | 33 |
| 2d | 17.1 | 66 |
| 3a | 12.7 | 55 |
| 3b | 13.8 | 60 |
| 3c | 7.3 | 37 |
| 3d | 19.1 | 69 |
3.5. Biological Tests
4. Conclusions
Acknowledgments
References
- Connolly, J.; Hill, R. Triterpenoids. Nat. Prod. Rep. 2008, 25, 794–830. [Google Scholar] [CrossRef]
- Huang, M.-T.; Ho, C.-H.; Wang, Z.Y.; Ferraro, T.; Lou, Y.-R.; Stauber, K.; Ma, W.; Georgiadis, C.; Laskin, J.D.; Conney, A.H. Inhibition of Skin Tumorigenesis by Rosemary and Its Constituents Carnosol and Ursolic Acid. Cancer Res. 1994, 54, 701–708. [Google Scholar]
- Vechia, L.D.; Gnoatto, S.C.B.; Gosmann, G. Oleanane and ursane derivatives and their importance on the discovery of potential antitumour, antiinflammatory and antioxidant drugs. Quim. Nova 2009, 32, 1245–1252. [Google Scholar] [CrossRef]
- Maia, J.L.; Lima-Junior, R.C.P.; David, J.P.; David, J.M.; Santos, F.A.; Rao, V.S. Oleanolic Acid, a Pentacyclic Triterpene Attenuates the Mustard Oil-Induced Colonic Nociception in Mice. Biol. Pharm. Bull. 2006, 29, 82–85. [Google Scholar] [CrossRef]
- Maia, J.L.; Lima-Junior, R.C.P.; Melo, C.M.; David, J.P.; David, J.M.; Campos, A.R.; Santos, F.A.; Rao, V.S. Oleanolic acid, a pentacyclic triterpene attenuates capsaicin-induced nociception in mice: Possible mechanisms. Pharmacol. Res. 2006, 54, 282–286. [Google Scholar] [CrossRef]
- Cechinel-Filho, V.; Yunes, R.A. Estrategies for obtaining pharmacologically active compounds from medicinal plants. Concepts about structural modification for improve the activity. Quim. Nova 1998, 21, 99–105. [Google Scholar] [CrossRef]
- Harley, R.M. A review of Eriope and Eriopdion (Labiatae). Hooker’s Icon. Pl. 1976, 38, 1–107. [Google Scholar]
- David, J.P.; da Silva, E.F.; de Moura, D.L.; Guedes, M.L.S.; Assunção, R.J.; David, J.M. Lignans and triterpenes from cytotoxic extract of Eriope blanchetii. Quim. Nova 2001, 24, 730–733. [Google Scholar]
- Santos, E.O.; Lima, L.S.; David, J.M.; Martins, L.C.; Guedes, M.L.S.; David, J.P. Podophyllotoxin and other aryltetralin lignans from Eriope latifolia and Eriope blanchetii. Nat. Prod. Res. 2011, 25, 1450–1453. [Google Scholar] [CrossRef]
- Tanachatchairatana, T.; Bremner, J.B.; Chokchaisiri, R.; Suksamrarn, A. Antimycobacterial activity of cinnamate-based esters of the triterpenes betulinic, oleanolic and ursolic acids. Chem. Pharm. Bull. 2008, 56, 194–198. [Google Scholar] [CrossRef]
- Kvasnica, M.; Sarek, J.; Klinotova, E.; Dzubakb, P.; Hajduchb, M. Synthesis of phthalates of betulinic acid and betulin with cytotoxic activity. Bioorg. Med. Chem. 2005, 13, 3447–3454. [Google Scholar] [CrossRef]
- Qian, K.; Nakagawa-Gotoa, K.; Yu, D.; Marris-Natschkea, S.L.; Nitz, T.J.; Kilgore, N.; Allaway, G.P.; Lee, K.-H. Anti-AIDS Agents 73: Structure-Activity Relationship Study and Asymmetric Synthesis of 3-O-Monomethylsuccinyl Betulinic Acid Derivatives. Bioorg. Med. Chem. Lett. 2007, 17, 6553–6557. [Google Scholar] [CrossRef]
- Honda, T.; Finlay, H.J.; Gribble, G.W.; Suh, N.; Sporn, M.B. New enone derivatives of oleanolic acid and ursolic acid as inhibitors of nitric oxide production in mouse macrophages. Bioorg. Med. Chem. Lett. 1997, 7, 1623–1628. [Google Scholar] [CrossRef]
- Mahato, S.B.; Kundu, A.P. 13C-NMR Spectra of Pentacyclic Triterpenoids—A compilation and some salient features. Phytochemistry 1994, 37, 1517–1575. [Google Scholar] [CrossRef]
- Anderson, J.E.; Goetz, A. A blind comparison of simple bench-top bioassays and human tumour cell cytotoxicities as antitumor prescreens. Phytochem. Anal. 1991, 2, 107–111. [Google Scholar] [CrossRef]
- Barreiros, A.L.B.S.; David, J.M.; David, J.P. Oxidative stress: Relations between the formation of reactive species and the organism’s defense. Quim. Nova 2006, 29, 113–123. [Google Scholar] [CrossRef]
- Barreiros, A.L.B.S.; David, J.M.; David, J.P. Antioxidant Phenylpropanoid Esters of Triterpenes from Dioclea lasiophylla. Pharm. Biol. 2004, 42, 36–38. [Google Scholar]
- Serrano, C.; Ortega, T.; Villar, A. Biological activity of traditional medicines from Spain and Guatemala. Artemia salina Bioassays: A revision. Phytother. Res. 1996, 10, S118–S120. [Google Scholar]
- Aguiar, R.M.; Alves, C.Q.; David, J.M.; e Luciano S. Lima, L.C.R.; David, J.P.; de Queiróz, L.P. Antioxidant activities of isolated compounds from stems of Mimosa invisa Mart. ex Colla. Quim. Nova 2012, 35, 567–570. [Google Scholar] [CrossRef]
- Fontana, R.; Mendes, M.A.; Souza, B.M.; Donno, K.; César, L.M.M.; Palma, M.S. Jelleines: A family of antimicrobial peptides from the royal jelly of honeybees (Apis mellifera). Peptides 2004, 27, 2624–2631. [Google Scholar]
- Sample Availability: Samples of the compounds 1, 2, 3, 1a–d, 2a–d and 3a–d are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Silva, M.D.L.e.; David, J.P.; Silva, L.C.R.C.; Santos, R.A.F.; David, J.M.; Lima, L.S.; Reis, P.S.; Fontana, R. Bioactive Oleanane, Lupane and Ursane Triterpene Acid Derivatives. Molecules 2012, 17, 12197-12205. https://doi.org/10.3390/molecules171012197
Silva MDLe, David JP, Silva LCRC, Santos RAF, David JM, Lima LS, Reis PS, Fontana R. Bioactive Oleanane, Lupane and Ursane Triterpene Acid Derivatives. Molecules. 2012; 17(10):12197-12205. https://doi.org/10.3390/molecules171012197
Chicago/Turabian StyleSilva, Maria De L. e, Juceni P. David, Lidércia C. R. C. Silva, Rauldenis A. F. Santos, Jorge M. David, Luciano S. Lima, Pedro S. Reis, and Renato Fontana. 2012. "Bioactive Oleanane, Lupane and Ursane Triterpene Acid Derivatives" Molecules 17, no. 10: 12197-12205. https://doi.org/10.3390/molecules171012197