2. Results and Discussion
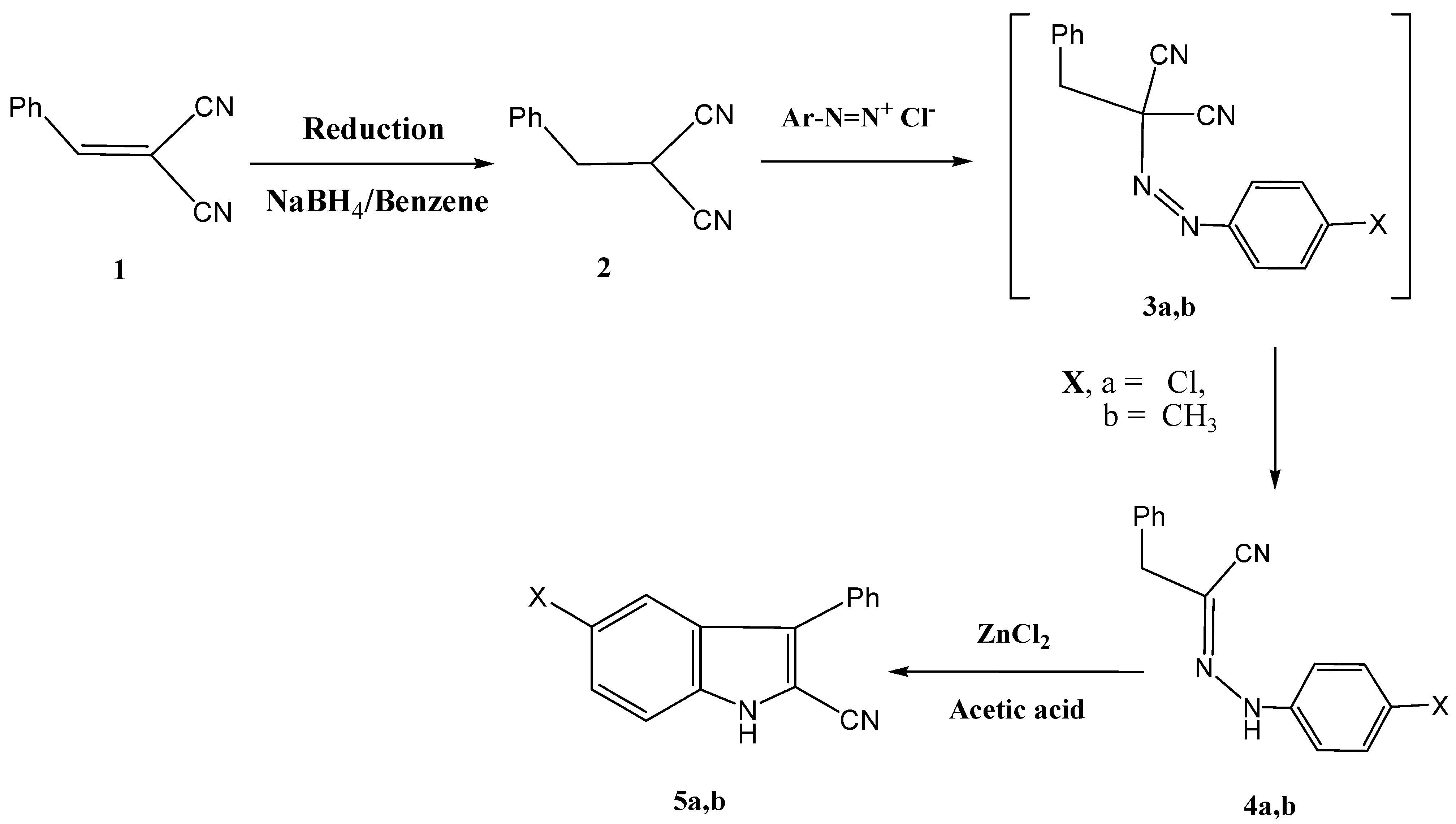
The hydrazononitriles
4a,
b were synthesized by reducing benzylidenemalononitrile (
1) with sodium borohydride as recently described, to yield
2 [
17]. Coupling of compound
2 with aromatic diazonium salts afforded intermediates
3. It is believed that the initially formed
3a,
b readily undergo Japp-Klingmann cleavage [
18] yielding the final isolable products
4a,
b in 75%, and 70% yield respectively. Compounds
4a,
b afforded the 2-cyanoindoles
5a,
b upon treatment with zinc chloride and glacial acetic acid. This is an example of the utility of the Fisher indole synthesis in the synthesis of 2-cyanoindoles (
Scheme 1).
Scheme 1.
Synthesis of indole-2-carbonitriles 5a,b.
Scheme 1.
Synthesis of indole-2-carbonitriles 5a,b.
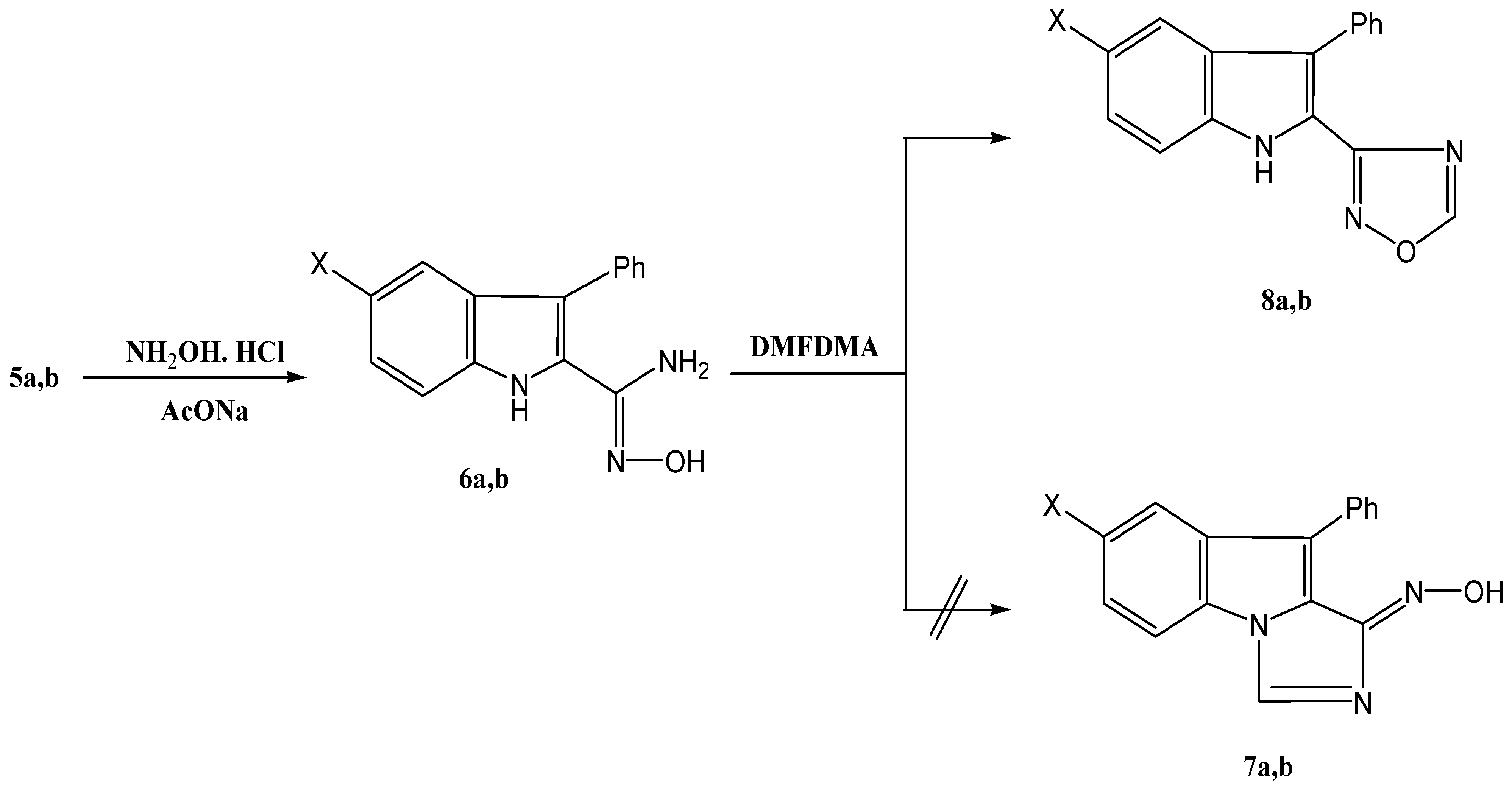
The 3-phenylindole-2-carbonitriles
5a,
b reacted with hydroxylamine hydrochloride to yield amidoximes
6a,
b. Reacting these products with dimethylformamide dimethylacetal (DMFDMA) afforded products
8a,
b in 68%, and 65% yield respectively, rather than
7, as indicated by a NOE experiment that showed an interaction between the indole-H-1, at 10.4 ppm and indole-H-7, at 6.8–7.3 ppm (
Scheme 2).
Scheme 2.
Synthesis of 1,2,4-oxadiazolylindole derivatives 8a,b.
Scheme 2.
Synthesis of 1,2,4-oxadiazolylindole derivatives 8a,b.
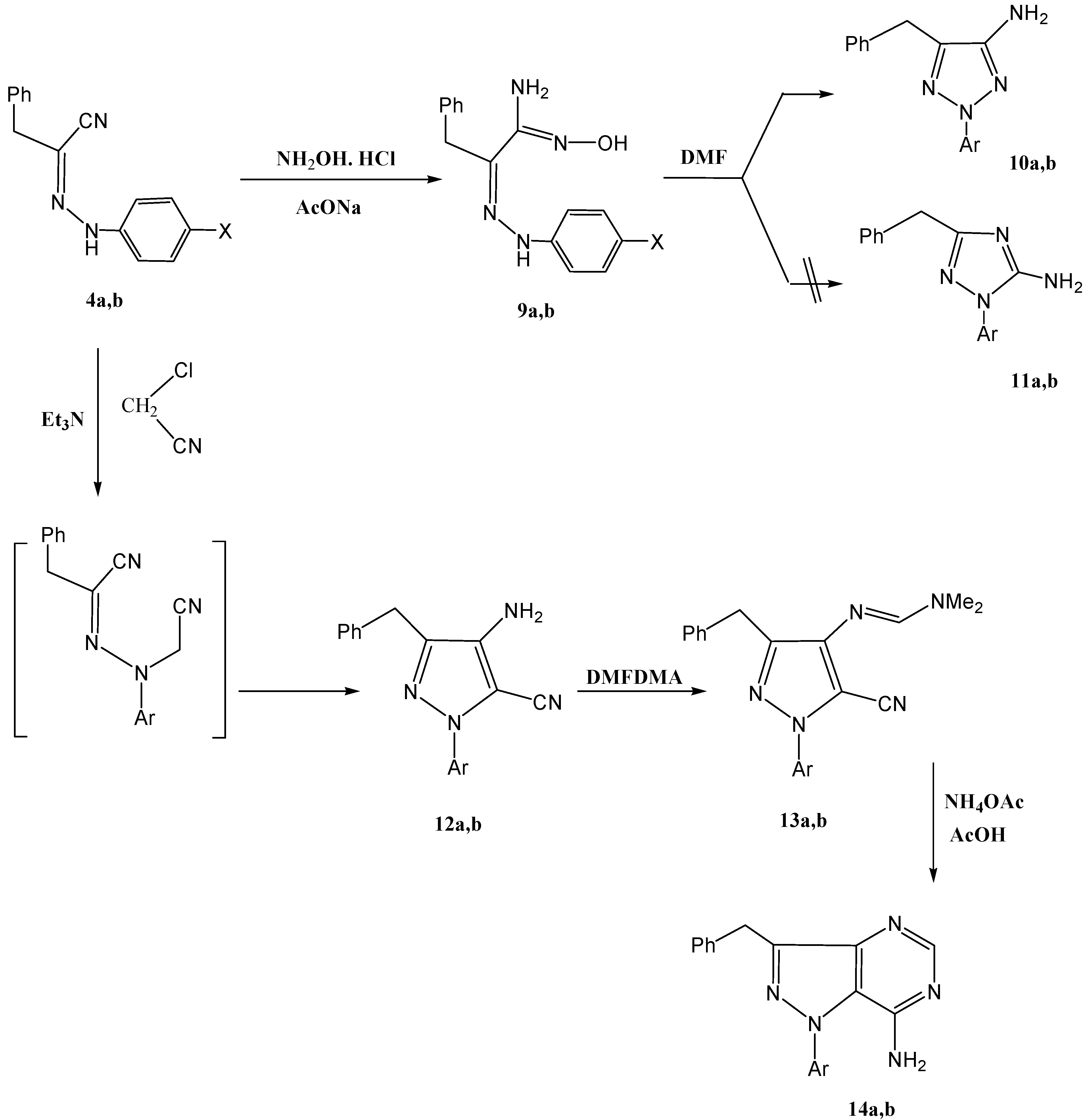
Our attention then shifted to explore the utility of 2-arylhydrazonals as efficient precursors to 1,2,3-triazoles. Compounds
4a,
b reacted with hydroxylamine hydrochloride to yield amidoximes
9a,
b that could be cyclized into
10a,
b or the isomeric
11a,
b upon reflux in DMF. From the previously reported findings concerning this reaction, the structure of the product is not clear, where the 1,2,3-triazole
10a,
b found a parallel in results reported for similar reactions under similar conditions [
1,
2,
3]. Although cyclization into isoxazoles has been reported by either refluxing of amidoximes in acidic medium [
4] or refluxing an ester derivative of an amidoxime in dimethylformamide [
19], cyclization to a 1,2,4-triazole via a Tiemann-like rearrangement has been reported by us in one case [
5]. Structures
11a,
b could be excluded due to the absence of any interaction between the NH
2 protons and the aryl protons in a NOE experiment (
Scheme 3). Moreover, we successfully confirmed that the correct structures are the 1,2,3-triazoles
10a,
b based on the obtained single crystal X-ray crystallography results recently reported by our group [
6].
Compounds
4a,
b was refluxed with chloroacetonitrile to yield
12a,
b that were then refluxed with DMFDMA to give the expected amidines
13a,
b. The amidines, so formed, were then cyclized in the presence of NH
4OAc and glacial acetic acid to give pyrazolo[4,3-
d]pyrimidines
14a,
b (
cf.
Scheme 3). The structure of the products
14a,
b was confirmed by the spatial interaction between the NH
2 protons, at 5.87 ppm, and aryl protons, at 7.08–7.17 ppm, in the NOE experiment.
Scheme 3.
Synthesis of 1,2,3-triazoles and pyrazolo[4,3-d]pyrimidines.
Scheme 3.
Synthesis of 1,2,3-triazoles and pyrazolo[4,3-d]pyrimidines.
3. Experimental
3.1. General Procedures
Melting points were recorded on a Gallenkamp apparatus and are uncorrected. Infrared spectra (KBr) were determined on a Jasco FT/IR-6300 FT-IR instrument. NMR measurements were determined on a Bruker DPX spectrometer at 600 MHz for 1H-NMR and 125 MHz for 13C-NMR, in DMSO-d6 as solvent and using TMS as internal standard. Mass spectra were measured on GC MS DFS-hermo spectrometers. Elemental analyses were measured by means of an Elementar Vario Micro Cube. Microwave heating was carried out with a single mode cavity Explorer Microwave Synthesizer (CEM Corporation, NC, USA), producing continuous irradiation and equipped with simultaneous external air−cooling system.
3.2. Synthesis of 2-Benzylidenemalononitrile (2)
This was prepared by the literature procedure [
17]. A mixture of benzaldehyde (10 mmol) and malononitrile (0.66 g, 10 mmol) was dissolved in aqueous ethanol (1:4, 25 mL) and stirred overnight. The reaction was followed to completion by TLC. To the pre-cooled reaction mixture, an equivalent amount of NaBH
4 was added portionwise with stirring at 0 °C for 15 min. The mixture was acidified with aqueous HCl and the product was extracted with CH
2Cl
2. The clear filtrate was evaporated under reduced pressure, and the remaining solid was collected by filtration. The solid product was then recrystallized from ethanol to give a colorless powder (82%); mp 85–86 °C (lit. mp 86–88 °C [
20]); IR (KBr):
υ = 2188.4 (CN), 2198 (CN) cm
–1;
1H-NMR:
δ = 3.28 (d,
J = 7.0 Hz, 2H), 3.88 (t,
J = 7.0 Hz, 1H), 7.32–7.44 (m, 5H, phenyl);
13C-NMR:
δ = 25.2 (CH), 37.1 (CH
2), 112.0 (2 CN), 128.6, 129.0, 129.2, 132.8 (aromatic carbons); MS,
m/z (%): 156.07 (M
+, 100), 77 (53); Anal. Calcd. for C
10H
8N
2: C, 76.90; H, 5.16; N, 17.94. Found: C, 76.77; H, 5.09; N, 17.72.
Coupling of 2 with aryldiazonium chlorides. A cold solution of the appropriate aryldiazonium salt was prepared by adding sodium nitrite solution (1.4 g dissolved in 10 mL water) to a pre-cooled solution of the corresponding arylamine hydrochloride (p-chloroaniline or p-toluidine, 10 mmol of arylamine in 6 mL 6 M HCl) with continuous stirring. The resulting aryldiazonium salt solutions were then added carefully to a cold ethanolic solution (50 mL) of benzylidenemalononitrile (2, 10 mmol) and sodium acetate trihydrate (2.8 g, 20 mmol). The mixture was stirred at room temperature for 1 h and the solid product formed was collected by filtration, washed with water and recrystallized from ethanol.
2-[(4-Chlorophenyl)hydrazono]-3-phenylpropionitrile (4a). This compound was obtained as pale yellow solid (75%); mp ~148 °C; IR (KBr): υ = 3300 (br. NH), 2185 (CN) cm–1; 1H-NMR: δ = 2.61 (s, 2H, CH2), 7.02 (d, 2H, J = 8 Hz, aryl), 7.34 (d, 2H, J = 8 Hz, aryl), 7.44 (m, 5H, phenyl), 8.9 (s, 1H, NH); 13C-NMR: δ = 30.2 (CH2), 113.6, 117.6 (CN), 119.5, 125.3, 126.8, 128.0, 129.8, 133.0, 135.9 (aromatic carbons), 159.1 (C=N); MS, m/z (%): 269.1 (M+, 100), 77 (66); Anal. Calcd. for C15H12ClN3: C, 66.79; H, 4.48; Cl, 13.14; N, 15.58. Found: C, 66.68; H, 4.40; Cl, 13.05; N, 15.46.
3-Phenyl-2-(p-tolylhydrazono)propionitrile (4b). This compound was obtained as a yellow solid (70%); mp ~126 °C; IR (KBr): υ = 3320 (br. NH), 2189 (CN) cm–1; 1H-NMR: δ = 1.71 (s, 3H, CH3), 2.66 (s, 2H, CH2), 7.13 (d, 2H, J = 8 Hz, aryl), 7.18 (d, 2H, J = 8 Hz, aryl), 7.22 (m, 5H, phenyl), 11.5 (s, 1H, NH); 13C-NMR: δ = 36.2 (CH3), 38.6 (CH2), 117.4 (CN), 118.9, 123.5, 127.3, 129.2, 132.4, 134.6 (aromatic carbons), 157.5 (C=N); MS, m/z (%): 249.31 (M+, 100), 77 (54). Anal. Calcd. for C16H15N3: C, 77.08; H, 6.06; N, 16.85. Found: C, 76.97; H, 5.98; N, 16.77.
Cyclization of 4a,b in the presence of ZnCl2 and glacial acetic acid. A mixture of 4a,b (10 mmol), zinc chloride (1.34 g, 10 mmol), and glacial acetic acid (50 mL) was refluxed and followed by TLC till completion after 24 h. The reaction mixture was poured into an ice/water mixture and the solid product, thus formed, was then collected by filtration and recrystallized from ethanol.
5-Chloro-3-phenyl-1H-indole-2-carbonitrile (5a). This compound was obtained as a yellow solid (60%); mp ~212 °C; IR (KBr): υ = 3300 (br. NH), 2206 (CN) cm–1; 1H-NMR: δ = 7.03–7.26 (m, 8H, aryl & phenyl), 11.1 (s, 1H, NH); 13C-NMR: δ = 117.6 (CN), 119.8, 121.4, 122.9, 124.7, 128.4, 129.6, 130.5, 133.0, 137.9 139.1, 142.2 (aromatic carbons); MS, m/z (%): 252.05 (M+, 100), 77 (51); Anal. Calcd. for C15H9ClN2: C, 71.29; H, 3.59; Cl, 14.03; N, 11.09. Found: C, 71.15; H, 3.52; Cl, 13.84; N, 10.97.
5-Methyl-3-phenyl-1H-indole-2-carbonitrile (5b). This compound was obtained as a colorless solid (70%); mp ~168 °C; IR (KBr): υ = 3320 (br. NH), 2189 (CN) cm–1; 1H-NMR: δ = 1.81 (s, 3H, CH3), 6.87–7.33 (m, 8H, phenyl), 10.8 (s, 1H, NH); 13C-NMR: δ = 35.2 (CH3), 117.6 (CN), 120.6, 122.3, 122.6, 123.4, 127.0, 128.7, 129.4, 132.7, 132.8, 134.6, 139.8 (aromatic carbons); MS, m/z (%): 232.1 (M+, 100), 77 (48); Anal. Calcd. for C16H12N2: C, 82.73; H, 5.21; N, 12.06. Found: C, 82.68; H, 5.14; N, 11.95.
3.3. Synthesis of 1,2,4-Oxadiazolyl-indoles 8a,b
A mixture of 5a,b (10 mmol), hydroxylamine hydrochloride (0.69 g, 10 mmol), and sodium acetate (3 g, 25 mmol) in ethanol (25 mL) was refluxed for 5 h. The reaction mixture was poured into ice/water with stirring while a yellow solid separated and was then collected by filtration. The crude product was refluxed with DMFDMA for 6 h. The pure products 8a,b were purified by recrystallization from ethanol.
3-(5-Chloro-3-phenyl-1H-indol-2-yl)-1,2,4-oxadiazole (8a). Obtained as a pale yellow powder (68%); mp ~142 °C; IR (KBr): υ = 1586 (aromatic C=C) cm–1; 1H-NMR: δ = 6.84–7.31 (m, 9H, aromatic), 10.4 (s, 1H, NH, imidazole); 13C-NMR: δ = 112.3, 115.2, 121.2, 122.8, 123.4, 124.0, 127.9, 128.5, 129.1, 131.4, 133.8, 134.7, 137.9, 148.6 (aromatic carbons); MS, m/z (%): 295.1 (M+, 56), 77 (100); Anal. Calcd. for C16H10ClN3O: C, 64.98; H, 3.41; Cl, 11.99; N, 14.21. Found: C, 64.91; H, 3.36; Cl, 11.91; N, 14.13.
3-(5-Methyl-3-phenyl-1H-indol-2-yl)-1,2,4-oxadiazole (8b). Obtained as a yellow solid (65%); mp ~124 °C; IR (KBr): υ = 3100 (aromatic CH) cm–1; 1H-NMR: δ = 2.69 (s, 3H, CH3), 6.72–7.28 (m, 9H, aromatic), 10.1 (s, 1H, NH, imidazole); 13C-NMR: δ = 36.3 (CH3), 110.6, 112.7, 119.3, 121.7. 122.6, 122.9, 123.9, 127.4, 128.9, 129.6, 131.2, 132.6, 134.8 142.6 (aromatic carbons); MS, m/z (%): 275.1 (M+, 83), 77 (100); Anal. Calcd. for C17H13N3O: C, 74.17; H, 4.76; N, 15.26. Found: C, 74.09; H, 4.66; N, 15.13.
3.4. Synthesis of 1,2,3-Triazole Derivatives 10a,b
A mixture of 4a,b (10 mmol), hydroxylamine hydrochloride (0.69 g, 10 mmol), and sodium acetate (3 g, 25 mmol) was dissolved in ethanol (25 mL). The mixture was refluxed for 4 h. The reaction mixture was poured into ice/water with stirring while a yellow solid separated and was then collected by filtration. The crude product, so formed, was then refluxed in DMF for 5 h and the reaction mixture was poured into cold water. The products 10a,b were purified by crystallization from ethanol.
5-Benzyl-2-(4-chlorophenyl)-2H-1,2,3-triazol-4-amine (10a). It was obtained as a yellow solid (75%); mp >250 °C; IR (KBr): υ = 3330 (br. NH2) cm–1; 1H-NMR: δ = 3.65 (s, 2H, CH2), 6.87 (s, 2H, NH2), 7.01–7.23 (m, 9H, aromatic); 13C-NMR: δ = 33.1 (CH2), 104.8, 121.3, 122.7, 122.9, 125.7, 128.3, 129.1, 132.0, 134.6, 141.0 (aromatic carbon); MS, m/z (%): 284.08 (M+, 65), 77 (84); Anal. Calcd. for C15H13ClN4: C, 63.27; H, 4.60; Cl, 12.45; N, 19.68. Found: C, 63.18; H, 4.53; Cl, 12.34; N, 19.62.
5-Benzyl-2-(4-tolyl)-2H-1,2,3-triazol-4-amine (10b). It was obtained as a yellow solid (70%); mp ~197 °C; IR (KBr): υ = 3340 (br. NH2) cm–1; 1H-NMR: δ = 2.44 (s, 3H, CH3), 3.61 (s, 2H, CH2), 5.82 (s, 2H, NH2), 7.01–7.24 (m, 9H, aromatic); 13C-NMR: δ = 30.8 (CH3), 32.6 (CH2), 106.4, 119.6, 121.2, 122.7, 124.3, 128.1, 128.4, 131.8, 133.2, 139.1 (aromatic carbons); MS, m/z (%): 264.14 (M+, 46), 77 (100); Anal. Calcd. for C16H16N4: C, 72.70; H, 6.10; N, 21.20. Found: C, 72.57; H, 6.02; N, 21.13.
3.5. Cyclization of 4a,b with Chloroacetonitrile in the Presence of Et3N
A mixture of 4a,b (10 mmol), chloroacetonitrile (0.75 g, 10 mmol), and triethylamine (0.5 mL) was irradiated at 80 W for 5 min (final temperature 140 °C). The reaction mixture was poured into a HCl/water mixture and the solid product, so formed, was then collected by filtration and recrystallized from ethanol.
4-Amino-3-benzyl-1-(4-chlorophenyl)-1H-pyrazole-5-carbonitrile (12a). This compound was obtained as a yellow solid (67%); mp ~227 °C; IR (KBr): υ = 3350 (br. NH2), 2210 (CN) cm–1; 1H-NMR: δ = 3.86 (s, 2H, CH2), 6.87 (s, 2H, NH2), 7.01–7.26 (m, 9H, aromatic); 13C-NMR: δ = 31.6 (CH2), 117.8 (CN), 119.9, 121.5, 122.3, 125.1, 125.4, 127.2, 129.8, 132.6, 133.8, 135.1, 138.7 (aromatic carbons); MS, m/z (%): 308.08 (M+, 27), 77 (100); Anal. Calcd. for C17H13ClN4: C, 66.13; H, 4.24; Cl, 11.48; N, 18.15. Found: C, 66.04; H, 4.07; Cl, 11.33; N, 18.05.
4-Amino-3-benzyl-1-p-tolyl-1H-pyrazole-5-carbonitrile (12b). This compound was obtained as a yellow solid (70%); mp ~204 °C; IR (KBr): υ = 3330 (br. NH2), 2190 (CN) cm–1; 1H-NMR: δ = 2.27 (s, 3H, CH3), 3.62 (s, 2H, CH2), 6.41 (s, 2H, NH2), 6.94–7.21 (m, 9H, aromatic); 13C-NMR: δ = 35.9 (CH3), 38.1 (CH2), 117.7 (CN), 119.2, 121.1, 121.7, 124.2, 127.9, 128.6, 129.7, 131.2, 132.4, 134.0, 136.4 (aromatic carbons); MS, m/z (%): 288.14 (M+, 62), 77 (100); Anal. Calcd. for C18H16N4: C, 74.98; H, 5.59; N, 19.43. Found: C, 74.88; H, 5.47; N, 19.27.
3.6. Synthesis of Pyrazolopyrimidine Derivatives
A mixture of 12a,b (10 mmol) and dimethylformamide dimethylacetal (1.8 g, 15 mmol) in dry xylene (50 mL) was refluxed for 6 h. The reaction mixture was cooled and then the product, so formed, was refluxed with ammonium acetate (1.54 g, 20 mmol) and glacial acetic acid (25 mL) for 4 h. The reaction mixture was cooled and treated with petroleum ether whereby a yellowish solid precipitated and was collected by filtration. The pure product was obtained by crystallized from ethanol.
3-Benzyl-1-(4-chlorophenyl)-1H-pyrazolo[4,3-d]pyrimidin-7-amine (14a). This compound was obtained as a yellow solid (72%); mp >250 °C; IR (KBr): υ = 3350 (br. NH2) cm–1; 1H-NMR: δ = 3.36 (s, 2H, CH2), 5.87 (s, 2H, NH2), 7.08–7.17 (m, 9H, aromatic), 8.98 (s, 1H, CH pyrimidine); 13C-NMR: δ = 34.6 (CH2), 104.7, 110.4, 114.0, 119.1, 121.4, 123.9, 124.2, 127.3, 128.7, 130.2, 131.7, 134.3, 139.7 (aromatic carbons); MS, m/z (%): 335.09 (M+, 48), 77 (100); Anal. Calcd. for C18H14ClN5: C, 64.38; H, 4.20; Cl, 10.56; N, 20.86. Found: C, 64.26; H, 4.06; Cl, 10.39; N, 20.67.
3-Benzyl-1-p-tolyl-1H-pyrazolo[4,3-d]pyrimidin-7-amine (14b). This compound was obtained as a yellow solid (65%); mp >250 °C; IR (KBr): υ = 3330 (br. NH2) cm–1; 1H-NMR: δ = 2.84 (s, 3H, CH3), 3.17 (s, 2H, CH2), 5.61 (s, 2H, NH2), 6.86–7.19 (m, 9H, aromatic), 8.62 (s, 1H, CH pyrimidine); 13C-NMR: δ = 36.4 (CH3), 38.6 (CH2), 105.2, 112.4, 119.1, 119.7, 122.1, 124.9, 128.2, 128.6, 130.9, 132.3, 134.6, 135.0, 137.8 (aromatic carbons); MS, m/z (%): 315.1 (M+, 53), 77 (100); Anal. Calcd. for C19H17N5: C, 72.36; H, 5.43; N, 22.21. Found: C, 72.25; H, 5.31; N, 22.08.