Bioassays Against Pinewood Nematode: Assessment of a Suitable Dilution Agent and Screening for Bioactive Essential Oils
Abstract
:1. Introduction
2. Results and Discussion
2.1. Assessment of Triton X-100 and Acetone Nematicidal Activity
2.2. Comparative Evaluation of Essential Oils’ Nematicidal Activity Using Triton X-100 or Acetone as Solvent
| Species | Dilution agent | 0.25 mg/mL | 0.5 mg/mL | 1 mg/mL |
|---|---|---|---|---|
| Cymbopogon citratus | Triton X-100 * | 14.98 ± 2.17 | 81.60 ± 1.72 | − |
| Acetone | 83.80 ± 1.08 | 89.39 ± 1.18 | − | |
| Origanum vulgare | Triton X-100 * | 2.78 ± 0.68 | 3.72 ± 0.56 | 26.61 ± 3.83 |
| Acetone | 94.90 ± 1.06 | 98.81 ± 0.51 | 100.00 ± 0.00 | |
| Satureja montana 1 | Triton X-100 * | 7.13 ± 1.19 | − | − |
| Acetone | 57.60 ± 2.44 | − | − |
| Species | l | c | LC100 | |
|---|---|---|---|---|
| Origanum vulgare * | 0.100 ± 0.0017 a | 1.770 ± 0.095 a | 0.498 ± 0.028 a | |
| Ruta graveolens 1 | 0.096 ± 0.0014 a | 1.799 ± 0.052 a | 0.571 ± 0.046 b | |
| Ruta graveolens 2 | 0.095 ± 0.0008 a | 1.915 ± 0.135a | 0.663 ± 0.032 c | |
| Satureja montana 1 * | 0.099 ± 0.0003 a | 1.946 ± 0.008 a | 0.793 ± 0.002d | |
| Satureja montana 2 | 0.089 ± 0.0024 b | 2.832 ± 0.056 b | 0.819 ± 0.007 d | |
| Satureja montana 3 | 0.089 ± 0.0013 c | 2.798 ± 0.020 c | 0.828 ± 0.001d | |

2.3. Essential Oils Nematicide Activity
| Code | Family / Species | Collection place or source b | Date | Plant part c Status | I.P. d | Oil yield(%, v/w) | Mortality e (%) |
|---|---|---|---|---|---|---|---|
| Anacardiaceae | |||||||
| Scm | Schinus molle L. | Évora | 2005 | Leaves, Fresh | H | 0.40 | 1.54 ± 0.47 |
| Apiaceae | |||||||
| Al | Angelica lignescens Reduron et Danton | Flores (Az) | 2008 | AP (V), Fresh | H | 0.08 | 1.75 ± 0.47 |
| Cha | Chaerophyllum azoricum Trelease | Flores (Az) | 2008 | AP (V), Fresh | H | 0.25 | 1.20 ± 0.48 |
| Fv1 | Foeniculum vulgare Miller | Graciosa (Az) | 2008 | AP (F), Fresh | H | 0.33 | 6.21 ± 0.71 * |
| Fv2 | Foeniculum vulgare Miller | HS | 2008 | Seeds, Dried | H | 5.61 | 8.60 ± 0.81 * |
| Fv3 | Foeniculum vulgare Miller | HS | 2008 | Seeds, Dried | H | 6.09 | 9.89 ± 1.71 * |
| Fv4 | Foeniculum vulgare Miller | HS | 2008 | Seeds, Dried | H | 5.88 | 6.29 ± 0.91 * |
| Fv5 | Foeniculum vulgare Miller | BPGV | 2008 | Seeds, Dried | H | 4.78 | 5.95 ± 0.79 * |
| Fv6 | Foeniculum vulgare Miller | HS | 2008 | Seeds, Dried | H | 1.07 | 7.13 ± 0.50 * |
| Cupressaceae | |||||||
| Cj | Cryptomeria japonica (L. fil.) D. Don. f | Flores (Az) | 2008 | Berries, Fresh | H | 0.41 | 0.79 ± 0.39 |
| Jb1 | Juniperus brevifolia (Seub.) Antoine | Flores (Az) | 2008 | Berries, Fresh | H | 0.06 | 0.84 ± 0.16 * |
| Jb2 | Juniperus brevifolia (Seub.) Antoine | Flores (Az) | 2008 | AP (V), Fresh | H | 0.45 | 2.56 ± 0.66 |
| Geraniaceae | |||||||
| Pg1 | Pelargonium graveolens L’Hér. | Lisboa | 2009 | AP (V), Fresh | H | 0.19 | 74.79 ± 2.56 * |
| Lamiaceae | |||||||
| Mo | Melissa officinalis L. | HS | 2009 | AP (F), Fresh | H | 0.04 | 99.30 ± 0.54 * |
| Ma | Mentha aquatica L. | HS | 2009 | AP (F), Dried | H | 0.90 | 7.77 ± 0.83 * |
| Mc1 | Mentha cervina L. | Beja | 2005 | AP (F), Fresh | H | 2.00 | 93.56 ± 1.07 * |
| Mc2 | Mentha cervina L. | HS | 2009 | AP (V), Dried | H | 2.12 | 92.57 ± 1.48 * |
| Ms | Mentha spicata L. | Beja | 2009 | AP (V), Fresh | H | 0.25 | 47.36 ± 2.22 * |
| Nc | Nepeta cataria L. | HS | 2009 | AP (F), Fresh | H | 0.18 | 22.03 ± 2.66 * |
| Ov | Origanum vulgare L. a | Évora | 2007 | AP (F), Fresh | H | 1.70 | 100.00 ± 0.00 * |
| Ro1 | Rosmarinus officinalis L. | HS | 2009 | Leaves, Dried | H | 1.95 | 2.55 ± 0.84 |
| Ro2 | Rosmarinus officinalis L. | Lisboa | 2009 | AP (V), Fresh | H | 0.64 | 0.40 ± 0.41 |
| Ro3 | Rosmarinus officinalis L. | Lisboa | 2009 | AP (F), Fresh | H | 1.14 | 2.30 ± 0.52 |
| So1 | Salvia officinalis L. | Lisboa | 2009 | AP (V), Fresh | H | 0.54 | 1.06 ± 0.40 |
| So2 | Salvia officinalis L. | Lisboa | 2009 | AP (V), Fresh | H | 0.71 | 0.07 ± 0.43 |
| Sm1 | Satureja montana L.a | HS | 2008 | Leaves, Dried | H | 1.60 | 100.00 ± 0.00 * |
| Sm2 | Satureja montana L. | HS | 2009 | AP (V), Dried | H | 0.55 | 100.00 ± 0.00 * |
| Sm3 | Satureja montana L. | Beja | 2009 | AP (F), Fresh | D-E | − | 100.00 ± 0.00 * |
| Tc1 | Thymus caespititius Brot. | Madeira | 2006 | AP (F), Fresh | D-E | − | 6.06 ± 0.62 * |
| Tc2 | Thymus caespititius Brot. | S. Jorge (Az) | 2007 | AP (F), Fresh | D-E | − | 97.01 ± 0.98 * |
| Tc3 | Thymus caespititius Brot. | Flores (Az) | 2008 | AP (F), Fresh | H | 0.06 | 94.63 ± 1.30 * |
| Tc4 | Thymus caespititius Brot. | Corvo (Az) | 2008 | AP (F), Fresh | H | 0.22 | 99.44 ± 0.26 * |
| Tc5 | Thymus caespititius Brot. | Gerês | 2008 | AP (F), Fresh | H | 0.35 | 51.61 ± 3.60 * |
| Tc6 | Thymus caespititius Brot. | Graciosa (Az) | 2008 | AP (F), Fresh | H | 0.38 | 58.21 ± 2.19 * |
| Tca | Thymus camphoratus Hoffmans. & Link | Faro | 2008 | AP (F), Fresh | H | 0.21 | 3.30 ± 0.59 * |
| Tvl | Thymus villosus ssp. lusitanicus (Boiss.) Coutinho | Leiria | 2008 | AP (F), Fresh | H | 1.25 | 66.85 ± 3.44 * |
| Tzs | Thymus zygis ssp. sylvestris (Hoffmans. & Link) Coutinho | Leiria | 2008 | AP (F), Fresh | H | 0.23 | 24.25 ± 3.18 * |
| Lauraceae | |||||||
| Cc | Cinnamomum camphora (L.) T. Nees & C.H. Eberm. | Coimbra | 2009 | Branches without leaves, Dried | H | 0.55 | 1.56 ± 0.16 * |
| La | Laurus azorica (Seub.) J. Franco | Flores (Az) | 2008 | AP (V), Fresh | H | 0.25 | 2.17 ± 0.66 |
| Lnc1 | Laurus novocanariensis Rivas Mart., Lousã, Fern. Prieto, E. Díaz, J.C. Costa & C. Aguiar | Porto da Cruz, Madeira | 2009 | Branches, Fresh | H | 0.42 | 2.22 ± 0.39 * |
| Lnc2 | Laurus novocanariensis Rivas Mart., Lousã, Fern. Prieto, E. Díaz, J.C. Costa & C. Aguiar | Porto da Cruz, Madeira | 2009 | Branches, Fresh | H | 0.48 | 2.80 ± 0.34 * |
| Lnc3 | Laurus novocanariensis Rivas Mart., Lousã, Fern. Prieto, E. Díaz, J.C. Costa & C. Aguiar | Ribeiro Frio, Madeira | 2009 | Branches, Fresh | H | 0.39 | 2.66 ± 0.75 |
| Lnc4 | Laurus novocanariensis Rivas Mart., Lousã, Fern. Prieto, E. Díaz, J.C. Costa & C. Aguiar | Ribeiro Frio, Madeira | 2009 | Branches, Fresh | H | 0.64 | 2.91 ± 0.44 * |
| Lnc5 | Laurus novocanariensis Rivas Mart., Lousã, Fern. Prieto, E. Díaz, J.C. Costa & C. Aguiar | S. Vicente, Madeira | 2000 | Leaves, Fresh | H | 0.30 | 4.46 ± 0.54 * |
| Myrtaceae | |||||||
| Eg | Eucalyptus globulus Labill. | Lisbon | 2009 | AP (F), Fresh | H | 2.15 | 4.14 ± 0.85 * |
| Pittosporaceae | |||||||
| Pu1 | Pittosporum undulatum Vent. | Graciosa (Az) | 2008 | Berries, Fresh | H | 0.21 | 1.22 ± 0.34 |
| Pu2 | Pittosporum undulatum Vent. | Graciosa (Az) | 2008 | Leaves, Fresh | H | 0.08 | 1.46 ± 0.44 |
| Poaceae | |||||||
| Cyc | Cymbopogon citratus (DC) Stapf. a | Faro | 2008 | Leaves, Fresh | H | 0.80 | 98.86 ± 0.32 * |
| Rutaceae | |||||||
| Ca | Citrus aurantium L. | Évora | 2009 | Leaves, Fresh | H | 0.31 | 26.59 ± 1.47 * |
| Rg1 | Ruta graveolens L. | Évora | 2009 | AP (V), Fresh | H | 2.60 | 100.00 ± 0.00 * |
| Rg2 | Ruta graveolens L. | HS | 2009 | AP (F), Dried | H | 0.90 | 100.00 ± 0.00 * |
| Verbenaceae | |||||||
| Lc | Lippia citriodora Kunth | HS | 2009 | AP (V), Dried | H | 0.19 | 54.63 ± 3.53 * |
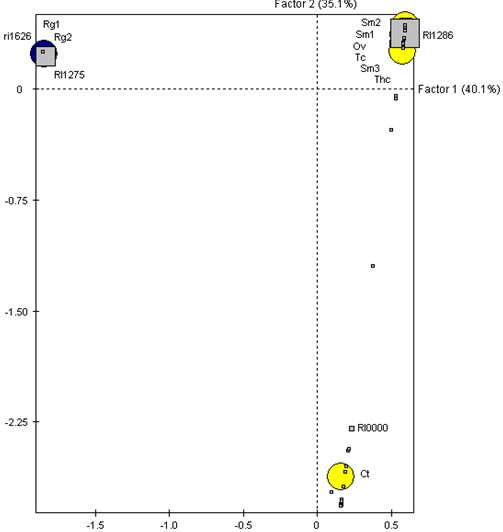
2.4. Chemical Profile of Essential Oils
| Lamiaceae | Rutaceae | ||||
|---|---|---|---|---|---|
| Compounds | RI a | Sm2 | Sm3 | Rg1 | Rg2 |
| 2-Methyloctane | 887 | − | − | t b | t |
| Tricyclene | 921 | t | t | − | − |
| α-Thujene | 924 | 0.3 | 2.4 | − | − |
| α-Pinene | 930 | 1.6 | 2.3 | − | − |
| Camphene | 938 | 1.6 | 0.1 | − | − |
| 1-Octen-3-ol | 961 | t | t | − | − |
| β-Pinene | 963 | 0.2 | 1.2 | − | − |
| n-Octanal | 973 | − | − | t | t |
| β-Myrcene | 975 | t | 2.7 | − | − |
| α-Phellandrene | 995 | t | 0.4 | − | − |
| δ-3-Carene | 1000 | t | 0.1 | − | − |
| α-Terpinene | 1002 | 0.3 | 4.1 | − | − |
| p-Cymene | 1003 | 20.3 | 8.1 | − | − |
| 1,8-Cineole | 1005 | t | t | − | − |
| β-Phellandrene | 1005 | t | 0.1 | − | − |
| Limonene | 1009 | 0.6 | 0.5 | − | − |
| cis-β-Ocimene | 1017 | t | t | − | − |
| γ-Terpinene | 1035 | 4.3 | 41.1 | − | − |
| trans-Sabinene hydrate | 1037 | t | t | − | − |
| 2-Nonanone | 1058 | − | − | t | t |
| 2,5-Dimethyl styrene | 1059 | t | t | − | − |
| Terpinolene | 1064 | 0.4 | t | − | − |
| cis-Sabinene hydrate | 1066 | t | t | − | − |
| n-Nonanal | 1073 | − | − | t | t |
| Linalool | 1074 | t | t | − | − |
| Geigerene isomer | 1116 | − | − | t | t |
| Geigerene | 1121 | − | − | 0.5 | 0.1 |
| Borneol | 1134 | 3.9 | 0.1 | − | − |
| Terpinen-4-ol | 1148 | 2.3 | 0.2 | − | − |
| α-Terpineol | 1159 | t | t | − | − |
| 2-Decanone | 1166 | − | − | t | t |
| Carvacrol methyl ether | 1224 | 3.7 | t | − | − |
| 2-Undecanone | 1275 | − | − | 94.4 | 92.8 |
| Thymol | 1275 | 15.2 | t | − | − |
| Carvacrol | 1286 | 40 | 35.3 | − | − |
| β-Bourbonene | 1379 | t | t | − | − |
| 2-Dodecanone c | 1389 | − | − | t | t |
| β-Caryophyllene | 1414 | 2.6 | 1.1 | − | − |
| β-Copaene | 1426 | t | t | − | − |
| Aromadendrene | 1428 | 0.3 | t | − | − |
| α-Humulene | 1447 | t | t | − | − |
| 2-Tridecanone | 1479 | − | − | t | t |
| β-Bisabolene | 1500 | 0.1 | t | − | − |
| trans-Calamenene | 1505 | 0.1 | t | − | − |
| δ-Cadinene | 1505 | 0.1 | 0.1 | − | − |
| β-Caryophyllene oxide | 1561 | 0.6 | t | − | − |
| UI Rg d | 1626 | − | − | 5.1 | 7.1 |
| % of identification | 98.6 | 99.9 | 94.9 | 92.9 | |
| Grouped components | |||||
| Monoterpene hydrocarbons | 29.6 | 63.1 | − | − | |
| Oxygen-containing monoterpenes | 65.2 | 35.6 | − | − | |
| Sesquiterpene hydrocarbons | 3.2 | 1.2 | − | − | |
| Oxygen-containing sesquiterpenes | 0.6 | − | − | − | |
| C13 compounds | − | − | 0.5 | 0.1 | |
| Others | − | − | 94.4 | 92.8 | |
3. Experimental
3.1. Plant Material
3.2. Essential Oils and Volatiles Extraction
3.3. Rearing and Collection of Nematodes
3.4. Bioassays
3.5. Determination of Essential Oils Composition
3.5.1. Gas Chromatography (GC)
3.5.2. Gas Chromatography-Mass Spectrometry (GC-MS)
3.6. Data Analysis
4. Conclusions
Acknowledgements
- Samples Availability: No pure compounds were used. Samples of the tested essential oils are available from the authors.
References
- Mota, M.; Vieira, P. Pine Wilt Disease: A Worldwide Threat to Forest Ecosystems; Springer: Heidelberg, Germany, 2008; pp. 1–3. [Google Scholar]
- Fonseca, L.; Lopes, A.; Cardoso, J.; Pestana, M.; Abreu, F.; Nunes, N.; Mota, M.; Abrantes, I. The Pinewood Nematode, Bursaphelenchus xylophilus. In Madeira Island. Presented at the Abstracts of the 30th International Symposium of European Society of Nematologists, Vienna, Austria, 19–23 September 2010; p. 176.
- Abelleira, A.; Picoaga, A.; Mansilla, J.P.; Aguin, O. Detection of Bursaphelenchus xylophilus, causal agent of pine wilt disease on Pinus pinaster in Northwestern Spain. Plant Dis. 2011, 95, 776. [Google Scholar]
- Portaria, N. 553-B/2008, Ministério da Agricultura, Desenvolvimento Rural e Pescas; Diário da República, Série 1, n. 123. In Imprensa Nacional Casa da Moeda, Lisboa, Portugal, 27 June 2008; pp. 4000(16)–4000(19).
- Alen, Y.; Nakajima, S.; Nitoda, T.; Baba, N.; Kanzaki, H.; Kawazu, K. Antinematodal activity of some tropical rainforest plants against the pinewood nematode, Bursaphelenchus xylophilus. Z. Naturforsch. C 2000, 55, 295–299. [Google Scholar]
- Choi, I.H.; Shin, S.C.; Park, I.K. Nematicidal activity of onion (Allium cepa) oil and its components against the pine wood nematode (Bursaphelenchus xylophilus). J. Nematol. 2007, 9, 231–235. [Google Scholar] [CrossRef]
- Hong, L.; Li, G.; Zhou, W.; Wang, X.; Zhang, K. Screening and isolation of a nematicidal sesquiterpene from Magnolia grandiflora L. Pest Manag. Sci. 2007, 63, 301–305. [Google Scholar] [CrossRef]
- Barbosa, P.; Lima, A.S.; Vieira, P.; Dias, L.S.; Tinoco, M.T.; Barroso, J.G.; Pedro, L.G.; Figueiredo, A.C.; Mota, M. Nematicidal activity of essential oils and volatiles derived from Portuguese aromatic flora against the pinewood nematode, Bursaphelenchus xylophilus. J. Nematol. 2010, 42, 8–16. [Google Scholar]
- UNEP Chemicals (United Nations Environment Programme). Screening Information Datasets for High Volume Chemicals. 2007. Available online: http://www.chem.unep.ch/irptc/sids/OECDSIDS/sidspub.html accessed on 1 October 2012.
- Ademola, I.O.; Eloff, J.N. Ovicidal and larvicidal activity of Cassia alata leaf acetone extract and fractions on Haemonchus contortus: In vitro studies. Pharm. Biol. 2011, 49, 539–544. [Google Scholar] [CrossRef]
- Kong, J.O.; Lee, S.M.; Moon, Y.S.; Lee, S.G.; Ahn, Y.J. Nematicidal activity of plant essential oils against Bursaphelenchus xylophilus (Nematoda: Aphelenchoididae). J. Asia-Pacific Entomol. 2006, 9, 173–178. [Google Scholar]
- Park, I.K.; Kim, J.; Lee, S.G.; Shin, S.C. Nematicidal activity of plant essential oils and components from Ajowan (Trachyspermum ammi), Allspice (Pimenta dioica) and Litsea (Litsea cubeba) essential oils against pine wood nematode (Bursaphelenchus xylophilus). J. Nematol. 2007, 39, 275–279. [Google Scholar]
- Elbadri, G.A.; Lee, D.W.; Park, J.C.; Yu, W.B.; Choo, H.Y.; Lee, S.M.; Lim, T.H. Nematocidal screening of essential oils and herbal extracts against Bursaphelenchus xylophilus. Plant Pathol. J. 2008, 24, 178–182. [Google Scholar] [CrossRef]
- Kim, J.; Seo, S.M.; Lee, S.G.; Shin, S.C.; Park, I.K. Nematicidal Activity of Plant Essential oils and components from Coriander (Coriandrum sativum), Oriental Sweetgum (Liquidambar orientalis), and Valerian (Valeriana wallichii) essential oils against Pine Wood Nematode (Bursaphelenchus xylophilus). J. Agric. Food Chem. 2008, 56, 7316–7320. [Google Scholar] [CrossRef]
- Limpert, E.; Stahel, W.A.; Abbt, M. Log-normal distributions across the sciences: Keys and clues. BioScience 2001, 51, 341–352. [Google Scholar] [CrossRef]
- Takai, K.; Soejima, T.; Suzuki, T.; Kawazu, K. Development of a water-soluble preparation of emamectin benzoate and its preventative effect against the wilting of pot-grown pine trees inoculated with the pine wood nematode, Bursaphelenchus xylophilus. Pest Manag. Sci. 2001, 57, 463–466. [Google Scholar] [CrossRef]
- Weibull, W. A statistical distribution function of wide applicability. J.Appl. Mech. 1951, 18, 293–297. [Google Scholar]
- Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Salgueiro, L.; Miguel, M.G.; Faleiro, M.L. Portuguese Thymbra and Thymus species volatiles: Chemical composition and biological activities. Curr. Pharm. Des. 2008, 14, 3120–3140. [Google Scholar] [CrossRef]
- Park, I.K.; Park, J.Y.; Kim, K.H.; Choi, K.S.; Choi, I.H.; Kim, C.S.; Shin, S.C. Nematicidal activity of plant essential oils and components from garlic (Allium sativum) and cinnamon (Cinnamomum verum) oils against the pine wood nematode (Bursaphelenchus xylophilus). Nematology 2005, 7, 767–774. [Google Scholar] [CrossRef]
- Kim, J.; Seo, S.M.; Park, I.K. Nematicidal activity of plant essential oils and components from Gaultheria fragrantissima and Zanthoxylum alatum against the pine wood nematode, Bursaphelenchus xylophilus. Nematology 2011, 13, 87–93. [Google Scholar] [CrossRef]
- Carramiñana, J.J.; Rota, C.; Burillo, J.; Herrera, A. Antibacterial efficiency of Spanish Satureja montana essential oil against Listeria monocytogenes among natural flora in minced pork. J. Food Protect. 2008, 71, 502–508. [Google Scholar]
- Nogueira, J.C.; Diniz, M.F.; Lima, E.O. In vitro antimicrobial activity of plants in Acute Otitis Externa. Braz. J. Otorhinolaryn. 2008, 74, 118–124. [Google Scholar]
- Soković, M.; Glamočlija, J.; Marin, P.D.; Brkić, D.; van Griensven, L.J. Antibacterial effects of the essential oils of commonly consumed medicinal herbs using an in vitro model. Molecules 2010, 15, 7532–7546. [Google Scholar] [CrossRef]
- Giordani, R.; Regli, P.; Kaloustian, J.; Mikaïl, C.; Abou, L.; Portugal, H. Antifungal effect of various essential oils against Candida albicans. Potentiation of antifungal action of amphotericin B by essential oil from Thymus vulgaris. Phytother. Res. 2004, 18, 990–995. [Google Scholar] [CrossRef]
- Lazar-Baker, E.E.; Hetherington, S.D.; Ku, V.V.; Newman, S.M. Evaluation of commercial essential oil samples on the growth of postharvest pathogen Monilinia fructicola (G. Winter) Honey. Lett. Appl. Microbiol. 2011, 52, 227–232. [Google Scholar]
- Faleiro, L.; Miguel, G.; Gomes, S.; Costa, L.; Venâncio, F.; Teixeira, A.; Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G. Antibacterial and antioxidant activities of essential oils isolated from Thymbra capitata L. (Cav.) and Origanum vulgare L. J. Agric. Food Chem. 2005, 53, 8162–8168. [Google Scholar]
- Prieto, M.; Iacopini, P.; Cioni, P.; Chericoni, S. In vitro activity of the essential oils of Origanum vulgare, Satureja montana and their main constituents in peroxynitrite-induced oxidative processes. Food Chem. 2007, 104, 889–895. [Google Scholar] [CrossRef]
- Grosso, C.; Oliveira, A.C.; Mainar, A.M.; Urieta, J.S.; Barroso, J.G.; Palavra, A.M.F. Antioxidant activities of the supercritical and conventional Satureja montana extracts. J. Food Sci. 2009, 74, 713–717. [Google Scholar] [CrossRef]
- Feo, V.; Simone, F.; Senatore, F. Potential allelochemicals from the essential oil of Ruta graveolens. Phytochemistry 2002, 61, 573–578. [Google Scholar] [CrossRef]
- Soleimani, M.; Azar, P.A.; Saber-Tehranil, M.; Rustaiyan, A. Volatile composition of Ruta graveolens L. World Appl. Sci. J. 2009, 7, 124–126. [Google Scholar]
- Kong, J.O.; Park, I.K.; Choi, K.S.; Shin, S.C.; Ahn, Y.J. Nematicidal and propagation activities of thyme red and white oil compounds toward Bursaphelenchus xylophilus (Nematode: Parasitaphelenchidae). J. Nematol. 2007, 39, 237–242. [Google Scholar]
- Ntalli, N.G.; Manconi, F.; Leonti, M.; Maxia, A.; Caboni, P. Aliphatic ketones from Ruta chalepensis (Rutaceae) induce paralysis on root knot nematodes. J. Agric. Food Chem. 2011, 59, 7098–7103. [Google Scholar] [CrossRef]
- Council of Europe, European Pharmacopoeia, 7th ed; European Directorate for the Quality of Medicines: Strasbourg, France, 2010; p. 241.
- Viglierchio, D.R.; Schmitt, R.V. On the methodology of nematode extraction from field samples: Baermann funnel modifications. J. Nematol. 1983, 15, 438–444. [Google Scholar]
- Püntener, W. Manual for Field Trials in Plant Protection, 2nd ed; Ciba-Geigy: Basle, Switzerland, 1981. [Google Scholar]
- Marquardt, D.W. An algorithm for least-squares estimation of nonlinear parameters. J. Soc. Ind. Appl. Math. 1953, 11, 431–441. [Google Scholar]
- Dubey, S.D. Normal and Weibull distributions. Nav. Res. Logist. Q. 1967, 14, 69–79. [Google Scholar] [CrossRef]
- Bonner, F.T.; Dell, T.R. The Weibull function: A new method of comparing seed vigor. J. Seed Technol. 1976, 1, 96–103. [Google Scholar]
- Chew, V. Comparing treatment means: a compendium. HortScience 1976, 11, 348–357. [Google Scholar]
- Penas, A.C.; Dias, L.S.; Mota, M.M. Precision and selection of extraction methods of aphelenchid nematodes from maritime pine wood, Pinus pinaster L. J. Nematol. 2002, 34, 62–65. [Google Scholar]
- Ury, H.K. A comparison of four procedures for multiple comparisons among means (pairwise contrasts) for arbitrary simple sizes. Technometrics 1976, 18, 89–97. [Google Scholar] [CrossRef]
- Sokal, R.R.; Rohlf, F.J. Biometry. The Principles and Practice of Statistics in Biological Research, 3rd ed; Freeman: New York, NY, USA, 1995; p. 239. [Google Scholar]
- Lebart, L.; Morineau, A.; Piron, M. Statistique Exploratoire Multidimensionelle; Dunod: Paris, France, 2000; Volume 3éme ed, pp. 170–171. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Barbosa, P.; Faria, J.M.S.; Mendes, M.D.; Dias, L.S.; Tinoco, M.T.; Barroso, J.G.; Pedro, L.G.; Figueiredo, A.C.; Mota, M. Bioassays Against Pinewood Nematode: Assessment of a Suitable Dilution Agent and Screening for Bioactive Essential Oils. Molecules 2012, 17, 12312-12329. https://doi.org/10.3390/molecules171012312
Barbosa P, Faria JMS, Mendes MD, Dias LS, Tinoco MT, Barroso JG, Pedro LG, Figueiredo AC, Mota M. Bioassays Against Pinewood Nematode: Assessment of a Suitable Dilution Agent and Screening for Bioactive Essential Oils. Molecules. 2012; 17(10):12312-12329. https://doi.org/10.3390/molecules171012312
Chicago/Turabian StyleBarbosa, Pedro, Jorge M. S. Faria, Marta D. Mendes, Luís Silva Dias, Maria Teresa Tinoco, José G. Barroso, Luis G. Pedro, Ana Cristina Figueiredo, and Manuel Mota. 2012. "Bioassays Against Pinewood Nematode: Assessment of a Suitable Dilution Agent and Screening for Bioactive Essential Oils" Molecules 17, no. 10: 12312-12329. https://doi.org/10.3390/molecules171012312