Phenolic and Lignan Glycosides from the Butanol Extract of Averrhoa carambola L. Root
Abstract
:1. Introduction
2. Results and Discussion
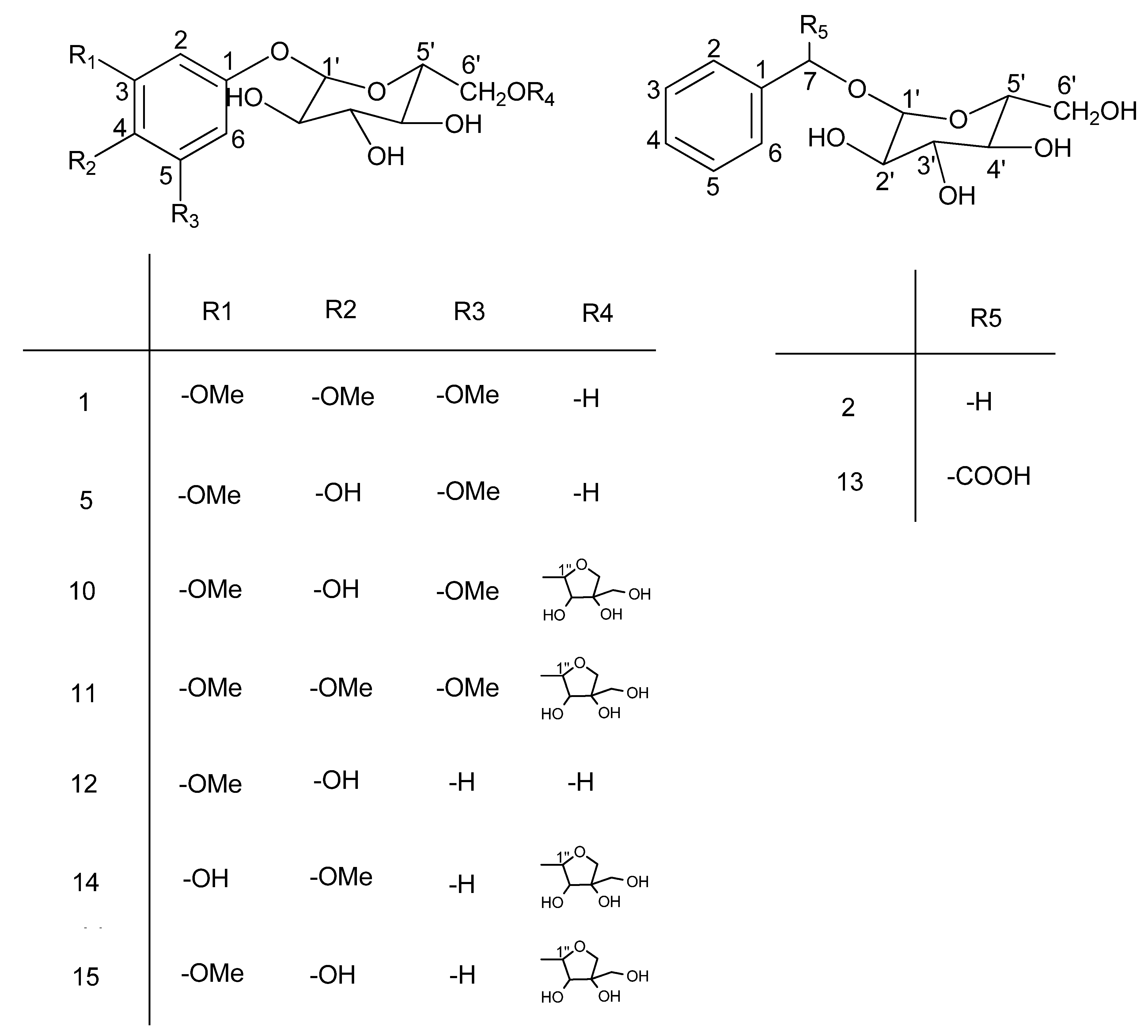

| C | 1 | 2 | 5 | 10 | 11 | 12 | 13 | 14 | 15 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 156.3 | 139.1 | 156.0 | 156.0 | 134.7 | 152.9 | 138.8 | 152.7 | 155.0 |
| 2 | 96.2 | 129.3 | 94.6 | 94.6 | 96.3 | 103.7 | 129.3 | 105.2 | 104.4 |
| 3 | 154.8 | 129.2 | 154.8 | 154.9 | 154.9 | 149.3 | 129.3 | 149.3 | 152.1 |
| 4 | 145.8 | 128.7 | 129.7 | 129.5 | 155.9 | 142.8 | 128.5 | 143.0 | 141.1 |
| 5 | 154.8 | 129.2 | 154.8 | 154.9 | 154.9 | 116.0 | 129.3 | 116.3 | 120.7 |
| 6 | 96.2 | 129.3 | 94.6 | 94.6 | 96.3 | 110.0 | 129.3 | 110.1 | 111.0 |
| 7 | - | 71.7 | - | - | - | - | 81.4 | - | - |
| R1 | 56.6 | - | 56.8 | 56.8 | 56.8 | 56.4 | - | - | 56.6 |
| R2 | 61.2 | - | - | - | 61.3 | - | - | 56.5 | - |
| R3 | 56.6 | - | 56.8 | 56.8 | 56.8 | - | - | - | - |
| R5 | - | - | - | - | - | 176.5 | - | - | |
| 1' | 103.2 | 103.3 | 106.2 | 105.1 | 103.0 | 103.7 | 103.5 | 103.6 | 104.4 |
| 2' | 75.0 | 75.2 | 75.8 | 74.9 | 74.9 | 75.0 | 75.1 | 74.9 | 75.0 |
| 3' | 78.2 | 78.0 | 78.3 | 77.8 | 77.2 | 78.2 | 78.3 | 77.7 | 78.1 |
| 4' | 71.7 | 71.8 | 71.4 | 71.3 | 71.5 | 71.6 | 71.4 | 71.4 | 71.7 |
| 5' | 78.4 | 78.0 | 77.8 | 75.6 | 77.8 | 78.1 | 78.0 | 77.3 | 77.0 |
| 6' | 61.2 | 62.8 | 62.6 | 69.3 | 67.0 | 62.5 | 62.6 | 70.1 | 68.7 |
| 1'' | - | - | - | 105.9 | 105.9 | - | - | 103.9 | 111.0 |
| 2'' | - | - | - | 77.5 | 77.2 | - | - | 77.3 | 77.8 |
| 3'' | - | - | - | 77.8 | 77.8 | - | - | 77.9 | 80.6 |
| 4'' | - | - | - | 74.9 | 74.9 | - | - | 74.9 | 75.1 |
| 5'' | - | - | - | 66.8 | 61.3 | - | - | 66.8 | 65.7 |
| C | 3 * | 4 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|
| 1 | 33.7 | 33.8 | 33.8 | 33.8 | 33.6 | 33.6 |
| 2 | 39.1 | 39.6 | 40.6 | 41.3 | 41.1 | 41.1 |
| 2a | 64.6 | 64.9 | 66.2 | 66.2 | 65.6 | 65.5 |
| 3 | 45.8 | 45.9 | 46.7 | 46.6 | 45.3 | 45.4 |
| 3a | 68.9 | 69.6 | 71.5 | 72.0 | 70.7 | 70.8 |
| 4 | 47.9 | 47.9 | 42.8 | 43.2 | b | b |
| 5 | 116.8 | 117.4 | 148.7 | 148.7 | 117.3 | 117.4 |
| 6 | 145.2 | 146.0 | 138.9 | 138.9 | 145.3 | 146.0 |
| 7 | 146.5 | 147.4 | 147.6 | 147.6 | 147.4 | 147.3 |
| 8 | 112.1 | 112.5 | 107.9 | 107.8 | 112.4 | 112.5 |
| 9 | 128.6 | 128.9 | 130.2 | 130.2 | 129.2 | 129.2 |
| 10 | 133.9 | 134.0 | 126.4 | 126.2 | 133.7 | 133.8 |
| 1' | 137.1 | 138.8 | 139.4 | 139.5 | 138.0 | 138.8 |
| 2' | 107.8 | 114.4 | 107.0 | 107.1 | 108.0 | 114.0 |
| 3' | 148.6 | 148.7 | 149.0 | 149.0 | 149.3 | 149.0 |
| 4' | 135.1 | 145.0 | 134.5 | 134.6 | 133.7 | 145.3 |
| 5' | 148.6 | 116.1 | 149.0 | 149.0 | 149.3 | 116.0 |
| 6' | 107.8 | 123.2 | 107.0 | 107.1 | 108.0 | 123.5 |
| 1'' | 105.1 | 105.2 | 104.8 | 104.3 | 103.9 | 103.9 |
| 2'' | 75.1 | 75.2 | 75.2 | 75.1 | 75.1 | 75.0 |
| 3'' | 77.4 | 77.9 | 77.9 | 78.0 | 78.0 | 77.9 |
| 4'' | 71.8 | 71.7 | 71.7 | 71.6 | 71.5 | 71.5 |
| 5'' | 78.0 | 78.2 | 78.2 | 78.2 | 78.2 | 78.3 |
| 6'' | 63.0 | 62.5 | 62.8 | 62.7 | 62.5 | 62.5 |
| 5-OCH3 | - | - | 60.2 | 60.1 | - | - |
| 7-OCH3 | 56.1 | 56.4 | 56.6 | 56.6 | 56.4 | 56.3 |
| 3'-OCH3 | 56.7 | 56.5 | 56.9 | 56.9 | 56.9 | 56.6 |
| 5'-OCH3 | 56.7 | - | 56.9 | 56.9 | 56.9 | - |
3. Experimental
3.1. General
3.2. Plant Materials
3.3. Extraction and Isolation
 −28.0 (c 0.3, MeOH), IR (KBr) cm−1: 3274.0, 2924.4, 1602.8, 1507.8, 1466.4, 1419.2, 1384.7, 1228.3, 1197.1, 1166.7, 1127.9, 1074.9, 1055.5, 1036.7, 1019.1, 998.9, 835.0, 819.7, 781.4. 1H-NMR (MeOD) δ: 3.42–3.47 (4H, m, overlaped, H-2', 3', 4', 5'), 3.66 (1H, dd, J = 12.1, 6.7 Hz, H-6'a), 3.70 (3H, s, 4-OMe), 3.81 (6H, s, 3, 5-OMe), 3.92 (1H, dd, J = 12.1, 2.4 Hz, H-6'b), 4.82 (1H, d, J = 7.3 Hz, H-1'), 6.49 (2H, s, H-2, 6) [11].
−28.0 (c 0.3, MeOH), IR (KBr) cm−1: 3274.0, 2924.4, 1602.8, 1507.8, 1466.4, 1419.2, 1384.7, 1228.3, 1197.1, 1166.7, 1127.9, 1074.9, 1055.5, 1036.7, 1019.1, 998.9, 835.0, 819.7, 781.4. 1H-NMR (MeOD) δ: 3.42–3.47 (4H, m, overlaped, H-2', 3', 4', 5'), 3.66 (1H, dd, J = 12.1, 6.7 Hz, H-6'a), 3.70 (3H, s, 4-OMe), 3.81 (6H, s, 3, 5-OMe), 3.92 (1H, dd, J = 12.1, 2.4 Hz, H-6'b), 4.82 (1H, d, J = 7.3 Hz, H-1'), 6.49 (2H, s, H-2, 6) [11]. −41.0 (c 0.2, MeOH), IR (KBr) cm−1: 3273.6, 2937.6, 1604.1, 1507.8, 1466.5, 1228.6, 1196.9, 1128.4, 1074.9, 1018.4, 999.3, 669.1. 1H-NMR (MeOD) δ: 7.42 (2H, d, J = 7.4 Hz, H-2, 6), 7.26–7.34 (3H, m, overlapped, H-3, 4, 5), 4.93 (1H, d, J = 11.8 Hz, H-7a), 4.68 (1H, d, J = 11.8 Hz, H-7b), 4.36 (1H, d, J = 7.7 Hz, H-1'), 3.90 (1H, dd, J = 2.2, 12.0 Hz, H-6'a), 3.68 (1H, dd, J = 5.6, 12.0 Hz, H-6'b) [12].
−41.0 (c 0.2, MeOH), IR (KBr) cm−1: 3273.6, 2937.6, 1604.1, 1507.8, 1466.5, 1228.6, 1196.9, 1128.4, 1074.9, 1018.4, 999.3, 669.1. 1H-NMR (MeOD) δ: 7.42 (2H, d, J = 7.4 Hz, H-2, 6), 7.26–7.34 (3H, m, overlapped, H-3, 4, 5), 4.93 (1H, d, J = 11.8 Hz, H-7a), 4.68 (1H, d, J = 11.8 Hz, H-7b), 4.36 (1H, d, J = 7.7 Hz, H-1'), 3.90 (1H, dd, J = 2.2, 12.0 Hz, H-6'a), 3.68 (1H, dd, J = 5.6, 12.0 Hz, H-6'b) [12]. +11.0 (c 0.1, MeOH), IR (KBr) cm−1: 3459.6, 2901.8, 1591.6, 1513.6, 1464.4, 1321.4, 1280.3, 1226.1, 1120.0, 1020.7, 949.3, 930.9. 1H-NMR (acetone-d6) δ: 7.18 (1H, s, H-8), 7.06 (1H, s, H-5), 6.54 (2H, s, H-2', 6'), 4.69 (1H, d, J = 3.7 Hz, H-1''), 4.25 (1H, d, J = 3.7 Hz, H-3a), 4.18 (1H, d, J = 4.2 Hz, H-3b), 4.09 (1H, dd, J = 10.0, 2.5 Hz, H-1b), 3.79 (3H, s, OMe), 3.77 (6H, s, 3', 5'-OMe), 3.61–3.72 (4H, m, overlaped, H-2'', 3'', 4'', 5''), 3.57–3.55 (1H, t), 3.41–3.37 (1H, m), 3.33 (1H, dd, J = 9.4, 4.1 Hz, H-6a), 3.26–3.25 (3H, m), 3.15 (1H, dd, J = 10.0, 2.9 Hz, H-1a), 2.79 (1H, dd, J = 16.1, 4.8 Hz, H-6b), 2.08 (1H, m, H-3) [13].
+11.0 (c 0.1, MeOH), IR (KBr) cm−1: 3459.6, 2901.8, 1591.6, 1513.6, 1464.4, 1321.4, 1280.3, 1226.1, 1120.0, 1020.7, 949.3, 930.9. 1H-NMR (acetone-d6) δ: 7.18 (1H, s, H-8), 7.06 (1H, s, H-5), 6.54 (2H, s, H-2', 6'), 4.69 (1H, d, J = 3.7 Hz, H-1''), 4.25 (1H, d, J = 3.7 Hz, H-3a), 4.18 (1H, d, J = 4.2 Hz, H-3b), 4.09 (1H, dd, J = 10.0, 2.5 Hz, H-1b), 3.79 (3H, s, OMe), 3.77 (6H, s, 3', 5'-OMe), 3.61–3.72 (4H, m, overlaped, H-2'', 3'', 4'', 5''), 3.57–3.55 (1H, t), 3.41–3.37 (1H, m), 3.33 (1H, dd, J = 9.4, 4.1 Hz, H-6a), 3.26–3.25 (3H, m), 3.15 (1H, dd, J = 10.0, 2.9 Hz, H-1a), 2.79 (1H, dd, J = 16.1, 4.8 Hz, H-6b), 2.08 (1H, m, H-3) [13]. +15.2 (c 0.32, MeOH), IR (KBr) cm−1: 3901.8, 3478.7, 1698.7, 1616.3, 1541.4, 1512.5, 1502.5, 1456.7, 1275.6, 1025.9. 1H-NMR (MeOD) δ: 6.79 (1H, d, J = 2.0 Hz, H-2'), 6.74 (1H, d, J = 8.0 Hz, H-5'), 6.65 (1H, br.s, H-8), 6.64 (1H, dd, J = 8.0, 2.0 Hz, H-6'), 4.12 (1H, d, H-3a), 4.07 (1H, t, H-3b), 3.81 (3H, s, OMe), 3.80 (3H, s, OMe), 3.77–3.70 (2H, m), 3.29–3.20 (4H, m, partly overlapped), 2.86–2.78 (2H, m, H-1a, H-1b), 2.10–2.07 (1H, m, H-3), 1.85–1.88 (1H, m, H-2) [13].
+15.2 (c 0.32, MeOH), IR (KBr) cm−1: 3901.8, 3478.7, 1698.7, 1616.3, 1541.4, 1512.5, 1502.5, 1456.7, 1275.6, 1025.9. 1H-NMR (MeOD) δ: 6.79 (1H, d, J = 2.0 Hz, H-2'), 6.74 (1H, d, J = 8.0 Hz, H-5'), 6.65 (1H, br.s, H-8), 6.64 (1H, dd, J = 8.0, 2.0 Hz, H-6'), 4.12 (1H, d, H-3a), 4.07 (1H, t, H-3b), 3.81 (3H, s, OMe), 3.80 (3H, s, OMe), 3.77–3.70 (2H, m), 3.29–3.20 (4H, m, partly overlapped), 2.86–2.78 (2H, m, H-1a, H-1b), 2.10–2.07 (1H, m, H-3), 1.85–1.88 (1H, m, H-2) [13]. −21.0 (c 0.25, MeOH), IR (KBr) cm−1: 3704.9, 2925.1, 1736.5, 1601.7, 1508.3, 1484.4, 1434.6, 1222.4, 1126.2, 1073.6, 996.1, 818.4, 639.0. 1H-NMR (MeOD) δ: 6.13 (2H, s, H-2, 6), 3.79 (6H, 2 × OMe), 4.66 (1H, d, J = 7.4 Hz, H-1'), 3.44 (1H, dd, J = 9.7, 2.3 Hz, H-2'), 3.40 (2H, dd, overlapped, H-3', 4'), 3.50-3.59 (1H, m, H-5'), 3.75 (1H, br. d, J = 17.3, 8.76 Hz, H-6'a), 3.67 (1H, dd, J = 11.8, 5.2 Hz, H-6'b) [14].
−21.0 (c 0.25, MeOH), IR (KBr) cm−1: 3704.9, 2925.1, 1736.5, 1601.7, 1508.3, 1484.4, 1434.6, 1222.4, 1126.2, 1073.6, 996.1, 818.4, 639.0. 1H-NMR (MeOD) δ: 6.13 (2H, s, H-2, 6), 3.79 (6H, 2 × OMe), 4.66 (1H, d, J = 7.4 Hz, H-1'), 3.44 (1H, dd, J = 9.7, 2.3 Hz, H-2'), 3.40 (2H, dd, overlapped, H-3', 4'), 3.50-3.59 (1H, m, H-5'), 3.75 (1H, br. d, J = 17.3, 8.76 Hz, H-6'a), 3.67 (1H, dd, J = 11.8, 5.2 Hz, H-6'b) [14]. +21.0 (c 0.4, MeOH), IR (KBr) cm−1: 3365.9, 2936.9, 1614.7, 1517.1, 1500.7, 1457.2, 1426.5, 1322.9, 1218.7, 1111.9, 900.8, 807.0, 632.7. 1H-NMR (C5D5N) δ: 6.73 (1H, s, H-8), 7.05 (2H, s, H-2', H-6'), 5.14 (1H, d, J = 6.1 Hz, H-4), 4.97 (1H, partly overlapped, H-1''), 4.52 (1H, dd, J = 11.7, 2.5 Hz, HA-2a or HA-3a), 4.42 (1H, dd, J = 10.0, 4.3 Hz, HB-2b or HB-3b), 4.36 (1H, d, J = 11.8, 5.3 Hz, HA-2a or HA-3a), 4.26–4.22 (2H, m, H-6''), 4.14–3.94 (4H, m, overlapped, H-2'',3'',4'',5''), 3.77 (3H, s, 7-OMe), 3.75 (3H, s, 5-OMe), 3.71 (6H, s,3, 5'-OMe), 3.14 (1H, dd, J = 14.7, 12.0 Hz, H-1a), 3.07 (1H, dd, J = 15.2, 4.4 Hz, H-1b ), 2.73 (1H, m, H-3), 2.14 (1H, m, H-2) [13,15].
+21.0 (c 0.4, MeOH), IR (KBr) cm−1: 3365.9, 2936.9, 1614.7, 1517.1, 1500.7, 1457.2, 1426.5, 1322.9, 1218.7, 1111.9, 900.8, 807.0, 632.7. 1H-NMR (C5D5N) δ: 6.73 (1H, s, H-8), 7.05 (2H, s, H-2', H-6'), 5.14 (1H, d, J = 6.1 Hz, H-4), 4.97 (1H, partly overlapped, H-1''), 4.52 (1H, dd, J = 11.7, 2.5 Hz, HA-2a or HA-3a), 4.42 (1H, dd, J = 10.0, 4.3 Hz, HB-2b or HB-3b), 4.36 (1H, d, J = 11.8, 5.3 Hz, HA-2a or HA-3a), 4.26–4.22 (2H, m, H-6''), 4.14–3.94 (4H, m, overlapped, H-2'',3'',4'',5''), 3.77 (3H, s, 7-OMe), 3.75 (3H, s, 5-OMe), 3.71 (6H, s,3, 5'-OMe), 3.14 (1H, dd, J = 14.7, 12.0 Hz, H-1a), 3.07 (1H, dd, J = 15.2, 4.4 Hz, H-1b ), 2.73 (1H, m, H-3), 2.14 (1H, m, H-2) [13,15]. −45.5 (c 0.3, MeOH), IR (KBr) cm−1: 3390.3, 2936.0, 1614.2, 1517.3, 1501.2, 1461.3, 1426.7, 1323.9, 1218.5, 1111.9, 901.0, 806.1, 626.2. 1H-NMR (MeOD) δ: 6.57 (1H, s, H-8), 6.41 (2H, s, H-2', H-6'), 4.22 (1H, d, J = 6.5 Hz, H-4), 4.13 (1H, d, J = 7.8 Hz, H-1"), 3.87–3.83 (5H, m), 3.85 (3H, s, OMe), 3.75 (6H, s, 2 × OMe), 3.69 (1H, dd, J = 11.9, 5.5 Hz, H-6"a). 3.63–3.57 (3H, m), 3.32–3.27 (m, mostly overlapped), 3.33 (3H, s, OMe), 3.22–3.14 (2H, m), 2.71–2.64 (2H, m, H-1a, H-1b), 2.15–2.11 (1H, m, H-3), 1.71–1.65 (1H, m, H-2) [13].
−45.5 (c 0.3, MeOH), IR (KBr) cm−1: 3390.3, 2936.0, 1614.2, 1517.3, 1501.2, 1461.3, 1426.7, 1323.9, 1218.5, 1111.9, 901.0, 806.1, 626.2. 1H-NMR (MeOD) δ: 6.57 (1H, s, H-8), 6.41 (2H, s, H-2', H-6'), 4.22 (1H, d, J = 6.5 Hz, H-4), 4.13 (1H, d, J = 7.8 Hz, H-1"), 3.87–3.83 (5H, m), 3.85 (3H, s, OMe), 3.75 (6H, s, 2 × OMe), 3.69 (1H, dd, J = 11.9, 5.5 Hz, H-6"a). 3.63–3.57 (3H, m), 3.32–3.27 (m, mostly overlapped), 3.33 (3H, s, OMe), 3.22–3.14 (2H, m), 2.71–2.64 (2H, m, H-1a, H-1b), 2.15–2.11 (1H, m, H-3), 1.71–1.65 (1H, m, H-2) [13]. −40.0 (c 0.5, MeOH), IR (KBr) cm−1: 3420.8, 2927.5, 1611.7, 1512.8, 1456.9, 1384.5, 1219.2, 1117.4. 1H-NMR (MeOD) δ: 6.68 (1H, s, H-8), 6.47 (2H, s, H-2', H-6'), 6.24 (1H, s, H-5), 4.08 (1H, d, J = 7.8 Hz, H-1"), 3.79–3.66 (6H, m), 3.83 (3H, s, OMe), 3.82 (6H, s, 2×OMe), 3.32–3.29 (2H, m), 3.19 (1H, dd, J = 8.9, 8.0 Hz), 3.08–3.06 (1H, m), 2.91 (1H, dd, J = 15.8, 10.3 Hz, H-1a), 2.77 (1H, dd, J = 16.0, 3.6 Hz, H-1b), 2.02–1.98 (2H, m, H-2, H-3) [13].
−40.0 (c 0.5, MeOH), IR (KBr) cm−1: 3420.8, 2927.5, 1611.7, 1512.8, 1456.9, 1384.5, 1219.2, 1117.4. 1H-NMR (MeOD) δ: 6.68 (1H, s, H-8), 6.47 (2H, s, H-2', H-6'), 6.24 (1H, s, H-5), 4.08 (1H, d, J = 7.8 Hz, H-1"), 3.79–3.66 (6H, m), 3.83 (3H, s, OMe), 3.82 (6H, s, 2×OMe), 3.32–3.29 (2H, m), 3.19 (1H, dd, J = 8.9, 8.0 Hz), 3.08–3.06 (1H, m), 2.91 (1H, dd, J = 15.8, 10.3 Hz, H-1a), 2.77 (1H, dd, J = 16.0, 3.6 Hz, H-1b), 2.02–1.98 (2H, m, H-2, H-3) [13]. −32.5 (c 0.1, MeOH), IR (KBr) cm−1: 3828.9, 3710.8, 2924.5, 2853.2, 1844.5, 1698.4, 1594.3, 1555.0, 1541.4, 1512.6, 1464.3, 1456.8, 1384.3, 1270.9, 1125.6, 1075.2, 1033.4. 1H-NMR (MeOD) δ: 6.74 (1H, d, J = 8.0 Hz, H-5'), 6.69 (1H, d, J = 1.9 Hz, H-2'), 6.65 (1H, br. s, H-8), 6.64 (1H, d, J = 2.0 Hz, H-6'), 6.19 (1H, s, H-5), 4.04 (1H, d, J = 7.8 Hz, H-1"). 3.85–3.63 (m), 3.81 (3H, s, OMe), 3.80 (3H, s, OMe), 3.35–3.25 (m, partly overlapped), 3.16 (1H, m), 3.06–3.04 (1H, m), 2.89–2.86 (1H, m, H-1a), 2.75 (1H, br. dd, J = 15.4, 4.0 Hz, H-1b), 1.97 (2H, m, H-2, H-3) [13].
−32.5 (c 0.1, MeOH), IR (KBr) cm−1: 3828.9, 3710.8, 2924.5, 2853.2, 1844.5, 1698.4, 1594.3, 1555.0, 1541.4, 1512.6, 1464.3, 1456.8, 1384.3, 1270.9, 1125.6, 1075.2, 1033.4. 1H-NMR (MeOD) δ: 6.74 (1H, d, J = 8.0 Hz, H-5'), 6.69 (1H, d, J = 1.9 Hz, H-2'), 6.65 (1H, br. s, H-8), 6.64 (1H, d, J = 2.0 Hz, H-6'), 6.19 (1H, s, H-5), 4.04 (1H, d, J = 7.8 Hz, H-1"). 3.85–3.63 (m), 3.81 (3H, s, OMe), 3.80 (3H, s, OMe), 3.35–3.25 (m, partly overlapped), 3.16 (1H, m), 3.06–3.04 (1H, m), 2.89–2.86 (1H, m, H-1a), 2.75 (1H, br. dd, J = 15.4, 4.0 Hz, H-1b), 1.97 (2H, m, H-2, H-3) [13]. −42.0 (c 0.6, MeOH), IR (KBr) cm−1: 3750.9, 3725.7, 3627.5, 3564.8, 2924.0, 1602.6, 1507.8, 1486.8, 1455.8, 1384.1, 1222.6, 1124.4, 1043.4, 818.3. 1H-NMR (MeOD) δ: 6.13 (2H, s, H-2, 6), 3.80 (6H, s, 3, 5-OMe), 4.24 (1H, d, J = 7.4 Hz, H-1'), 3.47–3.38 (6H, m, overlapped), 3.82 (1H, dd, J = 11.5, 5.3 Hz, H-6'a), 4.00 (1H, dd, J = 11.5, 2.1 Hz, H-6'b), 4.65 (1H, d, J = 7.7 Hz, H-1''), 3.76–3.72 (2H, m, overlapped) [16].
−42.0 (c 0.6, MeOH), IR (KBr) cm−1: 3750.9, 3725.7, 3627.5, 3564.8, 2924.0, 1602.6, 1507.8, 1486.8, 1455.8, 1384.1, 1222.6, 1124.4, 1043.4, 818.3. 1H-NMR (MeOD) δ: 6.13 (2H, s, H-2, 6), 3.80 (6H, s, 3, 5-OMe), 4.24 (1H, d, J = 7.4 Hz, H-1'), 3.47–3.38 (6H, m, overlapped), 3.82 (1H, dd, J = 11.5, 5.3 Hz, H-6'a), 4.00 (1H, dd, J = 11.5, 2.1 Hz, H-6'b), 4.65 (1H, d, J = 7.7 Hz, H-1''), 3.76–3.72 (2H, m, overlapped) [16]. −62.0 (c 0.8, MeOH), IR (KBr) cm−1: 3748.6, 3335.5, 1698.9, 1616.8, 1541.3, 1512.0, 1473.0, 1052.7. 1H-NMR (MeOD) δ: 6.47 (2H, s, H-2, 6), 3.82 (6H, s, 3, 5-OMe), 3.71 (3H, s, 4-OMe), 4.28 (1H, d, J = 7.6 Hz, H-1'), 4.82 (1H, d, J = 7.6 Hz, H-1''), 3.84 (2H, dd, J = 11.4, 5.3 Hz, H-4'') [17].
−62.0 (c 0.8, MeOH), IR (KBr) cm−1: 3748.6, 3335.5, 1698.9, 1616.8, 1541.3, 1512.0, 1473.0, 1052.7. 1H-NMR (MeOD) δ: 6.47 (2H, s, H-2, 6), 3.82 (6H, s, 3, 5-OMe), 3.71 (3H, s, 4-OMe), 4.28 (1H, d, J = 7.6 Hz, H-1'), 4.82 (1H, d, J = 7.6 Hz, H-1''), 3.84 (2H, dd, J = 11.4, 5.3 Hz, H-4'') [17]. −34.5 (c 0.15, MeOH), IR (KBr) cm−1: 3748.9, 3728.4, 2923.8, 1716.0, 1576.1, 1541.3, 1513.0, 1456.7, 1383.1, 1296.7, 1244.4, 1223.7, 1197.3, 1169.4, 1082.3, 1045.7, 989.4, 942.4, 840.1, 804.6. 1H-NMR (MeOD) δ: 6.80 (1H, d, J = 2.7 Hz, H-5), 6.69 (1H, d, J = 8.6 Hz, H-2), 6.59 (1H, dd, J = 8.6, 2.7 Hz, H-6), 3.83 (3H, s, OMe), 4.74 (1H, d, J = 7.4 Hz, H-1'), 3.46–3.35 (4H, overlapped), 3.90 (1H, dd, J = 12.0, 2.2 Hz, H-6'a), 3.69 (1H, dd, J = 12.0, 5.8 Hz, H-6'b) [18,19].
−34.5 (c 0.15, MeOH), IR (KBr) cm−1: 3748.9, 3728.4, 2923.8, 1716.0, 1576.1, 1541.3, 1513.0, 1456.7, 1383.1, 1296.7, 1244.4, 1223.7, 1197.3, 1169.4, 1082.3, 1045.7, 989.4, 942.4, 840.1, 804.6. 1H-NMR (MeOD) δ: 6.80 (1H, d, J = 2.7 Hz, H-5), 6.69 (1H, d, J = 8.6 Hz, H-2), 6.59 (1H, dd, J = 8.6, 2.7 Hz, H-6), 3.83 (3H, s, OMe), 4.74 (1H, d, J = 7.4 Hz, H-1'), 3.46–3.35 (4H, overlapped), 3.90 (1H, dd, J = 12.0, 2.2 Hz, H-6'a), 3.69 (1H, dd, J = 12.0, 5.8 Hz, H-6'b) [18,19]. −87.5 (c 0.4, MeOH), IR (KBr) cm−1: 3748.7, 3628.3, 3176.7, 2883.1, 1676.1, 1590.1, 1555.3, 1541.6, 1512.0, 1497.0, 1456.0, 1411.5, 1308.1, 1196.4, 1156.2, 1076.0, 897.1, 766.7, 702.8. 1H-NMR (MeOD) δ: 7.52 (2H, t, H-2, 6), 7.37–7.32 (3H, m, H-3, 4, 5), 5.29 (1H, s, H-7), 4.51 (1H, d, J = 7.8 Hz, H-1'), 3.87 (1H, d, J = 11.8 Hz, H-6'a), 3.67 (1H, dd, J = 12.0, 5.0 Hz, H-6'b), 3.46–3.40 (1H, m), 3.35 (1H, d, J = 8.0 Hz) [20,21].
−87.5 (c 0.4, MeOH), IR (KBr) cm−1: 3748.7, 3628.3, 3176.7, 2883.1, 1676.1, 1590.1, 1555.3, 1541.6, 1512.0, 1497.0, 1456.0, 1411.5, 1308.1, 1196.4, 1156.2, 1076.0, 897.1, 766.7, 702.8. 1H-NMR (MeOD) δ: 7.52 (2H, t, H-2, 6), 7.37–7.32 (3H, m, H-3, 4, 5), 5.29 (1H, s, H-7), 4.51 (1H, d, J = 7.8 Hz, H-1'), 3.87 (1H, d, J = 11.8 Hz, H-6'a), 3.67 (1H, dd, J = 12.0, 5.0 Hz, H-6'b), 3.46–3.40 (1H, m), 3.35 (1H, d, J = 8.0 Hz) [20,21]. −47.5 (c 0.3, MeOH), IR (KBr) cm−1: 3391.5, 1618.4, 1513.9, 1384.6, 1226.6, 1201.5, 1114.3, 1070.9, 856.2. 1H-NMR (MeOD) δ: 6.76 (1H, d, J = 9.0 Hz, H-5), 6.71 (1H, d, J = 2.4 Hz, H-2), 6.62 (1H, dd, J = 8.4, 3.0 Hz, H-6), 3.84 (3H, s, 4-OMe), 4.31 (1H, d, J = 7.2 Hz, H-1'), 3.61–3.58 (1H, m), 3.48–3.44 (1H, m), 3.79 (1H, dd, J = 11.4, 6.0 Hz, H-6'a), 4.10 (1H, dd, J = 11.4, 1.8 Hz, H-6'b), 4.74 (1H, d, J = 7.2 Hz, H-1''), 3.42–3.37 (3H, m, overlapped), 3.21–3.14 (3H, m, overlapped) [22].
−47.5 (c 0.3, MeOH), IR (KBr) cm−1: 3391.5, 1618.4, 1513.9, 1384.6, 1226.6, 1201.5, 1114.3, 1070.9, 856.2. 1H-NMR (MeOD) δ: 6.76 (1H, d, J = 9.0 Hz, H-5), 6.71 (1H, d, J = 2.4 Hz, H-2), 6.62 (1H, dd, J = 8.4, 3.0 Hz, H-6), 3.84 (3H, s, 4-OMe), 4.31 (1H, d, J = 7.2 Hz, H-1'), 3.61–3.58 (1H, m), 3.48–3.44 (1H, m), 3.79 (1H, dd, J = 11.4, 6.0 Hz, H-6'a), 4.10 (1H, dd, J = 11.4, 1.8 Hz, H-6'b), 4.74 (1H, d, J = 7.2 Hz, H-1''), 3.42–3.37 (3H, m, overlapped), 3.21–3.14 (3H, m, overlapped) [22]. −54.0 (c 0.3, MeOH), IR (KBr) cm−1: 3750.8, 3725.8, 3654.8, 3627.8, 2926.8, 1675.1, 1605.6, 1541.5, 1512.5, 1456.6, 1384.7, 1301.8, 1214.3, 1164.0, 1063.5, 954.2, 834.0, 807.0. 1H-NMR (MeOD) δ: 7.00 (1H, d, J = 8.7 Hz, H-2), 6.47 (1H, d, J = 2.8 Hz, H-5), 6.32 (1H, dd, J = 8.5, 2.8 Hz, H-6), 3.81 (3H, s, 3-OMe), 4.66 (1H, d, J = 7.5 Hz, H-1'), 3.43–3.42 (2H, m, overlapped), 3.48–3.45 (1H, m, overlapped), 3.62 (1H, dd, J = 11.1, 6.4 Hz, H-6'a), 3.98 (1H, dd, J = 11.1, 1.9 Hz, H-6'b), 4.98 (1H, d, J = 2.3 Hz, H-1''), 3.89 (1H, d, J = 2.3 Hz, H-2''), 3.74 (1H, d, J = 9.6 Hz, H-4''a), 3.93 (1H, d, J = 9.7 Hz, H-4''b), 3.58 (2H, s) [16].
−54.0 (c 0.3, MeOH), IR (KBr) cm−1: 3750.8, 3725.8, 3654.8, 3627.8, 2926.8, 1675.1, 1605.6, 1541.5, 1512.5, 1456.6, 1384.7, 1301.8, 1214.3, 1164.0, 1063.5, 954.2, 834.0, 807.0. 1H-NMR (MeOD) δ: 7.00 (1H, d, J = 8.7 Hz, H-2), 6.47 (1H, d, J = 2.8 Hz, H-5), 6.32 (1H, dd, J = 8.5, 2.8 Hz, H-6), 3.81 (3H, s, 3-OMe), 4.66 (1H, d, J = 7.5 Hz, H-1'), 3.43–3.42 (2H, m, overlapped), 3.48–3.45 (1H, m, overlapped), 3.62 (1H, dd, J = 11.1, 6.4 Hz, H-6'a), 3.98 (1H, dd, J = 11.1, 1.9 Hz, H-6'b), 4.98 (1H, d, J = 2.3 Hz, H-1''), 3.89 (1H, d, J = 2.3 Hz, H-2''), 3.74 (1H, d, J = 9.6 Hz, H-4''a), 3.93 (1H, d, J = 9.7 Hz, H-4''b), 3.58 (2H, s) [16].4. Conclusions
Supplementary Materials
Acknowledgments
- Samples Availability: Not available.
References
- Seidell, J.C. Obesity, Insulin Resistance and Diabetes-a Worldwide Epidemic. Br. J. Nutr. 2000, 83, S5–S8. [Google Scholar]
- Diez, J.J.; Iglesias, P. The role of the novel adipocyte derived hormone adiponectin in human disease. Eur. J. Endocrinol. 2003, 148, 293–300. [Google Scholar] [CrossRef]
- Huang, G.H.; Huang, R.B. Effects of Alcoholic Extracts of Averrhoa carambola L. Root on Blood Glucose Level and Lipid Peroxidation in Diabetic Mice. Lishizhen Med. Mater. Med. Res. 2009, 20, 2730–2731. [Google Scholar]
- Huang, G.H.; Huang, C.Z.; Huang, R.B. Effects of Averrhoa Carambola L. Root Polysaccharide on Serum Insulin and Index of Thymus, Spleen in STZ-induced Diabetic Mice. Chin. Pharm. 2009, 12, 848–850. [Google Scholar]
- Cazarolli, L.H.; Folador, P.; Moresco, H.H.; Brighente, I.M.; Pizzolatti, M.G.; Silva, F.R. Stimulatory effect of apigenin-6-C-β-L-fucopyranoside on insulin secretion and glycogen synthesis. Eur. J. Med. Chem. 2009, 44, 4668–4673. [Google Scholar] [CrossRef]
- Cazarolli, L.H.; Folador, P.; Moresco, H.H.; Brighente, I.M.; Pizzolatti, M.G.; Silva, F.R. Mechanism of action of the stimulatory effect of apigenin-6-C-(2''-O-α-L-rhamnopyranosyl)-β-L-fucopyranoside on 14C-glucose uptake. Chem. Biol. Interact. 2009, 179, 407–412. [Google Scholar] [CrossRef]
- Ranganayaki, S.; Singh, R.; Singh, A.K. The chemical examination of the bark of A. carambola. Proc. Natl. Acad. Sci. India A 1980, 50, 61–63. [Google Scholar]
- Gunasegaran, R. Flavonoids and anthocyanins of three Oxalidaceae. Fitoterapia 1992, 63, 89–90. [Google Scholar]
- Araho, D.; Miyakoshi, M.; Chou, W.; Kambara, T.; Mizutani, K.; Ikeda, T. A new flavone C-glycoside from the leaves of Averrhoa carambola. Nat. Med. 2005, 59, 113–116. [Google Scholar]
- Moresco, H.H.; Queiroz, G.S.; Pizzolatti, M.G.; Brighente, I. Chemical constituents and evaluation of the toxic and antioxidant activities of Averrhoa carambola leaves. Rev. Bras. Farmacogn. 2012, 22, 319–324. [Google Scholar] [CrossRef]
- Shimomura, H.; Sashida, Y.; Oohara, M.; Tenma, H. Phenolic Glucosides from Parabenzoin Praecox. Phytochemistry 1988, 27, 644–645. [Google Scholar] [CrossRef]
- Zeng, Q.; Chang, R.J.; Qin, J.J.; Cheng, X.G.; Zhang, W.D.; Jin, H.Z. New Glycosides from Dracocephalum tanguticum Maxim. Arch. Pharm. Res. 2011, 34, 2015–2020. [Google Scholar] [CrossRef]
- Achenbach, H.; Löwel, M.; Waibel, R.; Gupta, M.; Soils, P. New Lignan Glucosides from Stemmadenia minima. Planta Med. 1992, 58, 270–272. [Google Scholar] [CrossRef]
- Chung, M.I.; Lai, M.H.; Yen, M.H.; Wu, R.R.; Lin, C.N. Phenolics from Hypericum geminiflorum. Phytochemistry 1997, 44, 943–947. [Google Scholar] [CrossRef]
- Zhang, Z.Z.; Guo, D.A.; Li, C.L.; Zheng, J.H.; Kazuo, K.; Jia, Z.H.; Tamotsu, N. Studies on the lignan glycosides from gaultheria Yunnanensis. Yao Xue Xue Bao 1999, 34, 128–131. [Google Scholar]
- Kanchanapoom, T.; Kasai, R.; Yamasaki, K. Iridoid and phenolic diglycosides from Canthium berberidifolium. Phytochemistry 2002, 61, 461–464. [Google Scholar] [CrossRef]
- Kanchanapoom, T.; Kasai, R.; Yamasaki, K. Iridoid and phenolic glycosides from Morinda coreia. Phytochemistry 2002, 59, 551–556. [Google Scholar] [CrossRef]
- Gan, M.L.; Zhu, C.G.; Zhang, Y.L.; Zi, J.C.; Song, W.X.; Yang, Y.C.; Shi, J.G. Constituents from a water-soluble portion of ethanolic extract of Iodes cirrhosa. Zhongguo Zhong Yao Za Zhi 2010, 35, 456–466. [Google Scholar]
- Inoshiri, S.; Sasaki, M.; Kohda, H.; Otsuka, H.; Yamasaki, K. Aromatic glycosides from Berchemia racemosa. Phytochemistry 1987, 26, 2811–2814. [Google Scholar] [CrossRef]
- D’Abrosca, B.; DellaGreca, M.; Fiorentino, A.; Monaco, P.; Previtera, L.; Simonet, A.M.; Zarrelli, A. Potential allelochemicals from Sambucus nigra. Phytochemistry 2001, 58, 1073–1081. [Google Scholar] [CrossRef]
- Kitajima, J.; Tanaka, Y. Constituents of Prunus zippeliana leaves and branches. Chem. Pharm. Bull. 1993, 41, 2007–2009. [Google Scholar] [CrossRef]
- Arevalo, C.; Ruiz, I.; Piccinelli, A.L.; Campone, L.; Rastrelli, L. Phenolic Derivatives from the Leaves of Martinella obovata (Bignoniaceae). Nat. Prod. Comm. 2011, 6, 957–960. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wen, Q.; Lin, X.; Liu, Y.; Xu, X.; Liang, T.; Zheng, N.; Kintoko; Huang, R. Phenolic and Lignan Glycosides from the Butanol Extract of Averrhoa carambola L. Root. Molecules 2012, 17, 12330-12340. https://doi.org/10.3390/molecules171012330
Wen Q, Lin X, Liu Y, Xu X, Liang T, Zheng N, Kintoko, Huang R. Phenolic and Lignan Glycosides from the Butanol Extract of Averrhoa carambola L. Root. Molecules. 2012; 17(10):12330-12340. https://doi.org/10.3390/molecules171012330
Chicago/Turabian StyleWen, Qingwei, Xing Lin, Yeqi Liu, Xiaohui Xu, Tao Liang, Ni Zheng, Kintoko, and Renbin Huang. 2012. "Phenolic and Lignan Glycosides from the Butanol Extract of Averrhoa carambola L. Root" Molecules 17, no. 10: 12330-12340. https://doi.org/10.3390/molecules171012330




