Improving the Fermentation Production of the Individual Key Triterpene Ganoderic Acid Me by the Medicinal Fungus Ganoderma lucidum in Submerged Culture
Abstract
:1. Introduction
2. Results and Discussion
2.1. Production of GA-Me under Non-Optimized Culture Conditions
2.2. Optimization of GA-Me Production by CCD and RSM
2.2.1. Response Surface Analysis for the Optimization of Culture Condition Levels
| Independent variables | Range and levels | ||||
|---|---|---|---|---|---|
| −1.682 | −1 | 0 | 1 | 1.682 | |
| Glucose, x1 (g·L−1) | 36.59 | 40.0 | 45.0 | 50.0 | 53.41 |
| Peptone, x2 (g·L−1) | 3.32 | 4.00 | 5.0 | 6.00 | 6.68 |
| Fermentation time, x3 (h) | 265.91 | 300 | 13 | 400 | 434.09 |
| Runs | Coded values | YGA-Me (mg·L−1) | ||
|---|---|---|---|---|
| Factor x1 | Factor x2 | Factor x3 | ||
| 1 | −1 | −1 | −1 | 4.9 |
| 2 | −1 | −1 | 1 | 9.9 |
| 3 | −1 | 1 | −1 | 4.1 |
| 4 | −1 | 1 | 1 | 7.4 |
| 5 | 1 | −1 | −1 | 6.9 |
| 6 | 1 | −1 | 1 | 10.5 |
| 7 | 1 | 1 | −1 | 5.1 |
| 8 | 1 | 1 | 1 | 6.7 |
| 9 | −1.682 | 0 | 0 | 6.1 |
| 10 | 1.682 | 0 | 0 | 8.1 |
| 11 | 0 | −1.682 | 0 | 7.0 |
| 12 | 0 | 1.682 | 0 | 6.7 |
| 13 | 0 | 0 | −1.682 | 3.1 |
| 14 | 0 | 0 | 1.682 | 11.2 |
| 15 | 0 | 0 | 0 | 8.9 |
| 16 | 0 | 0 | 0 | 8.8 |
| 17 | 0 | 0 | 0 | 8.8 |
| 18 | 0 | 0 | 0 | 8.5 |
| 19 | 0 | 0 | 0 | 7.9 |
| 20 | 0 | 0 | 0 | 8.2 |
| Regression | DF | Sum of Squares | R-Square | F value | Pr > F |
|---|---|---|---|---|---|
| Linear | 3 | 63.2143 | 0.7614 | 34.16 | <0.0001 ** |
| Quadratic | 3 | 10.0708 | 0.1213 | 5.44 | 0.0177 * |
| Crossproduct | 3 | 3.5738 | 0.0430 | 1.93 | 0.1886 |
| Total model | 9 | 76.8589 | 0.9257 | 13.84 | 0.0002 ** |
| Factor | DF | Sum of Squares | Mean Square | F value | Pr > F |
|---|---|---|---|---|---|
| x1 | 4 | 8.3684 | 2.0921 | 3.39 | 0.0534 |
| x2 | 4 | 13.8737 | 3.4684 | 5.62 | 0.0123 * |
| x3 | 4 | 60.1597 | 15.0399 | 24.38 | <0.0001 ** |

| Parameter | DF | Coefficients estimated | Standard error | t value | Pr > |t| |
|---|---|---|---|---|---|
| Intercept | 1 | 8.5169 | 0.3203 | 26.59 | <0.0001 ** |
| x1 | 1 | 0.4586 | 0.2125 | 2.16 | 0.0563 |
| x2 | 1 | −0.6886 | 0.2125 | −3.24 | 0.0089 ** |
| x3 | 1 | 1.9860 | 0.2125 | 9.34 | <0.0001 ** |
| x1x1 | 1 | −0.5021 | 0.2069 | −2.43 | 0.0356 * |
| x1x2 | 1 | −0.2875 | 0.2777 | −1.04 | 0.3249 |
| x2x2 | 1 | −0.5905 | 0.2069 | −2.85 | 0.0171 * |
| x1x3 | 1 | −0.3875 | 0.2777 | −1.40 | 0.1931 |
| x2x3 | 1 | −0.4625 | 0.2777 | −1.67 | 0.1268 |
| x3x3 | 1 | −0.4844 | 0.2069 | −2.34 | 0.0412 * |
2.2.2. Interaction among the Factors and Selection of Their Optimum Levels
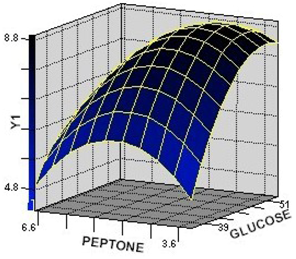
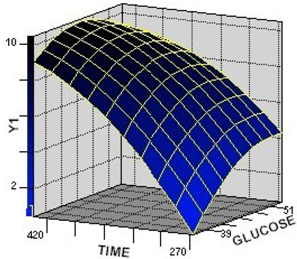
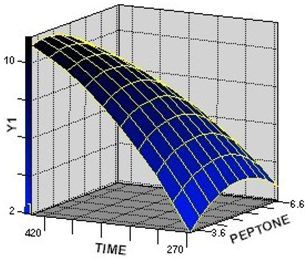
2.2.3. Verification of the Models in Flask Culture and Bioreactor Culture
| Culture systems | Cell density (g·L−1) | GA-Me content in Cell (μg/100 mg DW) | GA-Me production in Cell (mg·L−1) | GA-Me production in fermented broth (mg·L−1) | Final glucose concentration (g·L−1) |
|---|---|---|---|---|---|
| Flask culture | 11.3 ± 0.2 | 42.7 ± 0.4 | 4.8 ± 0.7 | 7.6 ± 0.2 | 4.7 ± 0.3 |
| Bioreactor culture | 9.1 ± 0.5 | 33.9 ± 0.6 | 3.1 ± 0.2 | 8.3 ± 0.7 | 4.3 ± 0.6 |
3. Experimental
3.1. Microorganism
3.2. Liquid Shake Culture
3.3. Bioreactor Culture
3.4. Determination of Dry Cell Weight
3.5. Measurement of GA-Me
3.6. RSM Experimental Design and Statistical Analysis

4. Conclusions
Acknowledgments
- Sample Availability: Not available.
References
- Liu, G.Q.; Zhang, K.C. Mechanisms of the anticancer action of Ganoderma lucidum (Leyss. ex. Fr.) Karst.: A new understanding. J. Integr. Plant Biol. 2005, 47, 129–135. [Google Scholar]
- Cheung, W.M.W.; Hui, W.S.; Chu, P.W.K.; Chiu, S.W.; Ip, N.Y. Ganoderma extract activates MAP kinases and induces the neuronal differentiation of rat pheochromocytoma PC12 cells. FEBS Lett. 2000, 486, 291–296. [Google Scholar] [CrossRef]
- Gao, J.J.; Min, B.S.; Ahn, E.M.; Nakamura, N.; Lee, H.K.; Hattori, M. New triterpene aldehydes, lucialdehydes A–C, from Ganoderma lucidum and their cytotoxicity against murine and human tumor cells. Chem. Pharm. Bull. 2002, 50, 837–840. [Google Scholar]
- Ha, T.B.T.; Gerhauser, C.; Zhang, W.D.; Ho-Chong-Line, N.; Fouraste, I. New lanostanoids from Ganoderma lucidum that induce NAD(P)H: Quinone oxidoreductase in cultured hepalclc7 murine hepatoma cells. Planta Med. 2000, 66, 681–684. [Google Scholar] [CrossRef]
- Gao, Y.H.; Zhou, S.F.; Jiang, W.Q.; Huang, M.; Dai, X.H. Effects of Ganopoly(R) (a Ganoderma lucidum polysaccharide extract) on the immune functions in advanced-stage cancer patients. Immunol. Invest. 2003, 32, 201–215. [Google Scholar] [CrossRef]
- Miyamoto, I.; Liu, J.; Shimizu, K.; Sato, M.; Kukita, A.; Kukita, T.; Kondo, R. Regulation of osteoclastogenesis by ganoderic acid DM isolated from Ganoderma lucidum. Eur. J. Pharmacol. 2009, 602, 1–7. [Google Scholar] [CrossRef]
- Chen, N.H.; Liu, J.W.; Zhong, J.J. Ganoderic acid T inhibits tumor invasion in vitro and in vivo through inhibition of MMP expression. Pharmacol. Rep. 2010, 62, 150–163. [Google Scholar] [CrossRef]
- Chen, Y.S.; Ming, S.S.; Cheng, T.W. Differential effects of ganodermic acid S on the thromboxane A2-signaling pathways in human platelets. Biochem. Pharmacol. 1999, 58, 587–595. [Google Scholar] [CrossRef]
- Kimura, Y.; Taniguchi, M.; Baba, K. Antitumor and antimetastatic effects on liver of triterpenoid fractions of Ganoderma lucidum: Mechanism of action and isolation of an active substance. Anticancer Res. 2002, 22, 3309–3318. [Google Scholar]
- Min, B.S.; Gao, J.J.; Nakamura, N.; Hattori, M. Triterpenes from the spores of Ganoderma lucidum and their cytotoxicity against meth-A and LLC tumor cells. Chem. Pharm. Bull. 2000, 48, 1026–1033. [Google Scholar] [CrossRef]
- Xu, J.W.; Zhao, W.; Zhong, J.J. Biotechnological production and application of ganoderic acids. Appl. Microbiol. Biotechnol. 2010, 87, 457–466. [Google Scholar] [CrossRef]
- Wang, G.; Zhao, J.; Liu, J.W.; Huang, Y.; Zhong, J.J.; Tang, W. Enhancement of IL-2 and IFN-γ expression and NK cells activity involved in the anti-tumor effect of ganoderic acid Me in vivo. Int. Immunopharmacol. 2007, 7, 864–870. [Google Scholar] [CrossRef]
- Chen, N.H.; Liu, J.W.; Zhong, J.J. Ganoderic acid Me inhibits tumor invasion through down-regulating matrix metalloproteinases 2/9 gene expression. J. Pharmacol. Sci. 2007, 108, 212–216. [Google Scholar]
- Chen, N.H.; Zhong, J.J. Ganoderic acid Me induces G1 arrest in wild-type p53 human tumor cells while G1/S transition arrest in p53-null cells. Process Biochem. 2009, 44, 928–933. [Google Scholar] [CrossRef]
- Komoda, Y.; Shimizu, M.; Sonoda, Y.; Sato, Y. Ganoderic acid and derivatives as cholesterol synthesis inhibitors. Chem. Pharm. Bull. 1989, 37, 531–533. [Google Scholar]
- Fang, Q.H.; Zhong, J.J. Two-stage culture process for improved production of ganoderic acid by liquid fermentation of higher fungus Ganoderman lucidum. Biotechnol. Progr. 2002, 18, 51–54. [Google Scholar] [CrossRef]
- Liu, G.Q.; Xiao, H.X.; Wang, X.L.; Zhao, Y.; Zhang, Y.G.; Ren, G.P. Stimulated production of triterpenoids of Ganoderma lucidum by an ether extract from the medicinal insect, Catharsius molossus and identification of the key stimulating active components. Appl. Biochem. Biotechnol. 2011, 165, 87–97. [Google Scholar] [CrossRef]
- Tang, Y.J.; Zhang, W.; Zhong, J.J. Performance analyses of a pH-shift and DOT-shift integrated fed-batch fermentation process for the production of ganoderic acid and Ganoderma polysaccharides by medicinal mushroom Ganoderma lucidum. Bioresource Technol. 2009, 100, 1852–1859. [Google Scholar] [CrossRef]
- Zhang, W.X.; Zhong, J.J. Effect of oxygen concentration in gas phase on sporulation and individual ganoderic acids accumulation in liquid static culture of Ganoderma lucidum. J.Biosci. Bioeng. 2010, 109, 37–40. [Google Scholar] [CrossRef]
- Wagner, R.; Mitchell, D.A.; Sassaki, G.L.; De Almeida Amazonas, M.A.L.; Berovic, M. Current techniques for the cultivation of Ganoderma lucidum for the production of biomass, ganoderic acid and polysaccharides. Food Technol. Biotechnol. 2003, 41, 371–382. [Google Scholar]
- Xu, P.; Ding, Z.Y.; Qian, Z.; Zhao, C.X.; Zhang, K.C. Improved production of mycelial biomass and ganoderic acid by submerged culture of Ganoderma lucidum SB97 using complex media. Enzyme Microb. Technol. 2008, 42, 325–331. [Google Scholar] [CrossRef]
- Xu, J.W.; Xu, Y.N.; Zhong, J.J. Production of individual ganoderic acids and expression of biosynthetic genes in liquid static and shaking cultures of Ganoderma lucidum. Appl. Microbiol. Biotechnol. 2010, 85, 941–948. [Google Scholar] [CrossRef]
- Liu, G.Q.; Wang, X.L. Optimization of critical medium components using response surface methodology for biomass and extracellular polysaccharide production by Agaricus blazei. Appl. Microbiol. Biotechnol. 2007, 74, 78–83. [Google Scholar] [CrossRef]
- Liu, G.Q.; Ding, C.Y.; Zhang, K.C. Effects of powdered dung beetle (Catharsius molossus) on cell growth and triterpenoid production of Ganoderma lucidum. Mycosystema 2008, 27, 757–762. [Google Scholar]
- Baskar, G.; Rajesh, L.K.S.; Pavithra, S.K.; Aarathi, S.; Renganathan, S. Production of ganoderic acid by Ganoderma lucidium MTCC 1039 from cottonseed oil cake, Statistical screening of process variables. Indian J. Biotechnol. 2011, 10, 65–69. [Google Scholar]
- Gong, H.G.; Zhong, J.J. Hydrodynamic shear stress affects cell growth and metabolite production by medicinal mushroom Ganoderma lucidum. Chin. J.Chem. Eng. 2005, 13, 426–428. [Google Scholar]
- The SAS Statistical Package, version 8.1; an integrated system of software; SAS Institute Inc.: Cary, NC, USA, 2000.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Liu, G.-Q.; Wang, X.-L.; Han, W.-J.; Lin, Q.-L. Improving the Fermentation Production of the Individual Key Triterpene Ganoderic Acid Me by the Medicinal Fungus Ganoderma lucidum in Submerged Culture. Molecules 2012, 17, 12575-12586. https://doi.org/10.3390/molecules171112575
Liu G-Q, Wang X-L, Han W-J, Lin Q-L. Improving the Fermentation Production of the Individual Key Triterpene Ganoderic Acid Me by the Medicinal Fungus Ganoderma lucidum in Submerged Culture. Molecules. 2012; 17(11):12575-12586. https://doi.org/10.3390/molecules171112575
Chicago/Turabian StyleLiu, Gao-Qiang, Xiao-Ling Wang, Wen-Jun Han, and Qin-Lu Lin. 2012. "Improving the Fermentation Production of the Individual Key Triterpene Ganoderic Acid Me by the Medicinal Fungus Ganoderma lucidum in Submerged Culture" Molecules 17, no. 11: 12575-12586. https://doi.org/10.3390/molecules171112575




