Growth Inhibition of Streptococcus from the Oral Cavity by α-Amyrin Esters
Abstract
:1. Introduction
2. Results and Discussion
2.1. Isolation of α- and β-Amyrin
2.2. Esterification of α-Amyrin
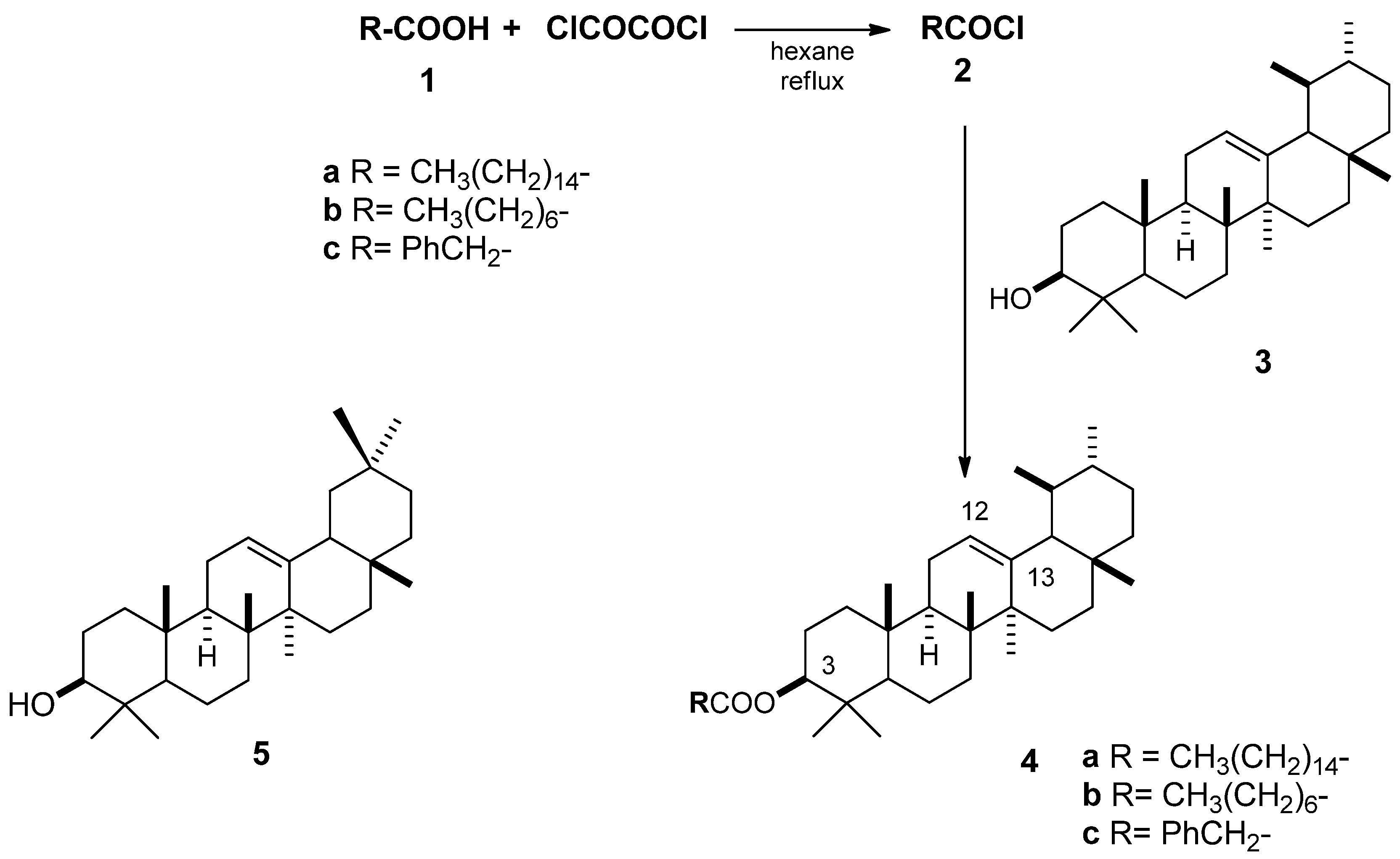
2.3. Antimicrobial Activity Using the Macrodilution Broth Method
| Compound | Terpene concentration (μg/mL) | ||||
|---|---|---|---|---|---|
| 10 | 53.33 | 100 | 533.33 | 1000 | |
| α-amyrin (3) | 62 | 46 | 25 | 25 | 16 |
| β-amyrin (5) | 72 | 61 | 58 | 46 | 37 |
| α-amyrin palmitate (4a) | 83 | 82 | 68 | 46 | 43 |
| α-amyrin octanoate (4b) | 89 | 62 | 60 | 40 | 39 |
| α-amyrin phenylacetate (4c) | 19 | 16 | 12 | 12 | 9 |
| Compound | S. salivarius | S. sanguinis | S. oralis |
|---|---|---|---|
| α-amyrin (3) | 68–56% | 56–20% | 95–47% |
| β-amyrin (5) | 68–57% | 90–63% | 100–46% |
| α-amyrin phenylacetate (4c) | 86–12% | 83–48% | 3–1% |
3. Experimental
3.1. General
3.2. Microorganisms
3.2.1. Inoculum Preparation
3.2.2. Statistical Analysis
3.3. Isolation of Triterpenes
3.4. Esterification of α-Amyrin
3.5. Macrodilution Broth Method for Growth Inhibition Determination
4. Conclusions
Acknowledgments
References
- Lavoie, J.M.; Stevanovic, T. Variation of chemical composition of the lipophilic extracts from yellow birch (Betula alleghaniensis) foliage. J. Agric. Food Chem 2005, 53, 4747–4756. [Google Scholar] [CrossRef]
- Singh, B.; Sahu, P.M.; Sharma, M.K. Anti-inflamatory and antimicrobial activities of triterpenoids from Strobilantes callosus Ness. Phytomedicine 2002, 9, 355–359. [Google Scholar] [CrossRef]
- Nagarajan, S.; Mohan Rao, L.J. Triterpenoids from swallow roots a convenient HPLC method for separation. J. Chromatogr. Sci. 2007, 45, 189–194. [Google Scholar]
- Hernandez-Vazquez, L.; Palazon, J.; Navarro-Ocaña, A. The Pentacyclic Triterpenes α, β-amyrins: A review of sources and Biological Activities. In Phytochemicals, A Global Perspective of the Role in Nutrition and Health; Venketeshwer, R., Ed.; InTech: Rijeka, Croatia, 2012; pp. 487–501. [Google Scholar]
- Pinto, H.S.A.; Pinto, L.M.S.; Cunha, G.M.A.; Chaves, M.H.; Santos, F.A.; Rao, V.S. Anti-inflammatory effect of α, β-amyrin, a pentacyclic triterpene from Protium heptaphyllum in rat model of acute periodontitis. Inflammopharmacology 2008, 16, 48–52. [Google Scholar] [CrossRef]
- Barros, F.W.; Bandeira, P.N.; Lima, D.J.; Meira, A.S.; de Farias, S.S.; Albuquerque, M.R.; dos Santos, H.S.; Lemos, T.L.; de Morais, M.O.; Costa-Lotufo, L.V.; et al. Amyrin esters induce cell death by apoptosis in HL-60 leukemia cells. Bioorg. Med. Chem. 2011, 19, 1268–1276. [Google Scholar]
- Johann, S.; Soldi, C.; Lyon, J.P.; Pizzolatti, M.G.; Resende, M.A. Antifungal activity of the amyrin derivatives and In vitro inhibition of Candida albicans adhesion to human epithelial. Lett. Appl. Microbiol. 2007, 45, 148–153. [Google Scholar]
- Singh, B.; Dubey, M.M. Estimation of Triterpenoids from Heliotropium marifolium Koen ex Retz. in vivo and in vitro. I. Antimicrobial screening. Phytother. Res. 2001, 15, 231–234. [Google Scholar] [CrossRef]
- Porto, S.T.; Rangel, R.; Furtado, A.J.C.N.; de Carvalho, T.C.; Martins, G.H.C.; Veneziani, C.S.R.; da Costa, B.F.; Vinholis, H.C.A.; Cunha, R.W.; Heleno, C.G.V.; et al. Pimarane-type diterpernes: Antimicrobial activity against oral pathogens. Molecules 2009, 14, 191–199. [Google Scholar] [CrossRef]
- Castillo, A.; Rubiano, S.; Gutiérrez, J.; Hermoso, A.; Liébana, J. Post-pH effect in oral streptococci. Clin. Microbiol. Infect. 2000, 6, 142–146. [Google Scholar] [CrossRef]
- Allaker, R.P.; Douglas, C.W. Novel anti-microbial therapies for dental plaque-related diseases. Int. J. Antimicrob. Agents 2009, 33, 8–13. [Google Scholar] [CrossRef]
- Sasaki, H.; Matsumoto, M.; Maeda, M.; Hamada, S.; Ooshima, T. Antibacterial activity of polyphenol components in oolong tea extract against Streptococus mutans. Caries Res. 2004, 38, 2–8. [Google Scholar] [CrossRef]
- Ferrazzano, G.F.; Amato, I.; Ingenito, A.; Zarreli, A.; Pinto, G.; Pollio, A. Plant polyphenols and their anti-cariogenic properties: A review. Molecules 2011, 16, 1486–1507. [Google Scholar]
- Ferrazzano, G.F.; Roberto, L.; Amato, I.; Cantile, T.; Sangianantonio, G.; Ingenito, A. Antimicrobial propierties of Green tea extract against cariogenic microflora: An in vivo study. J. Med. Food 2011, 14, 907–911. [Google Scholar] [CrossRef]
- Kubo, I.; Muroi, H.; Himejima, M. Antibacterial activity of totarol and its potentiation. J. Nat. Prod. 1992, 55, 1436–1440. [Google Scholar] [CrossRef]
- Rivero-Cruz, J.F.; Sánchez-Nieto, S.; Benítez, G.; Casimiro, X.; Ibarra-Alvarado, C.; Rojas-Molina, A.; Rivero-Cruz, B. Antibacterial compounds isolated from Byrsonima crassifolia. Rev. Latinoam. Quím. 2009, 37, 155–162. [Google Scholar]
- Hernández-Vázquez, L.; Mangas, S.; Palazón, J.; Navarro-Ocaña, A. Valuable medicinal plants and resins: Commercial phytochemicals with bioactive properties. Ind. Crops Prod. 2010, 31, 476–480. [Google Scholar] [CrossRef]
- Serreqi, A.N.; Leone, R.; del Rio, L.F.; Mei, S.; Fernandez, M.; Breuil, C. Identification and quantificacion of important Steryl esteres in Aspen Wood. J. Am. Oil Chem. Soc. 2000, 77, 413–419. [Google Scholar] [CrossRef]
- Zayka, L.L. Spices and herbs: their antimicrobial activity and its determination. J. Food Saf. 1988, 9, 97–118. [Google Scholar] [CrossRef]
- Van Houte, J. Role of micro-organisms in caries etiology. J. Dent. Res. 1994, 73, 672–681. [Google Scholar]
- Soldi, C.; Pizzolatti, M.G.; Luiz, A.P.; Marcon, R.; Meoti, F.C.; Mioto, L.A.; Santos, R.S. Synthetic derivatives of the α- and β-amirina triterpenes and their antinociceptive properties. Bioorg. Med. Chem. 2008, 16, 3377–3386. [Google Scholar]
- Soldi, C.; Pizzolatti, M.G.; Luiz, A.P.; Marcon, R.; Meoti, F.C.; Mioto, L.A.; Santos, R.S. Synthetic derivatives of the α- and β-amirina triterpenes and their antinociceptive properties. Bioorg. Med. Chem. 2008, 16, 3377–3386. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds α and β-amyrin are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Díaz-Ruiz, G.; Hernández-Vázquez, L.; Luna, H.; Wacher-Rodarte, M.D.C.; Navarro-Ocaña, A. Growth Inhibition of Streptococcus from the Oral Cavity by α-Amyrin Esters. Molecules 2012, 17, 12603-12611. https://doi.org/10.3390/molecules171112603
Díaz-Ruiz G, Hernández-Vázquez L, Luna H, Wacher-Rodarte MDC, Navarro-Ocaña A. Growth Inhibition of Streptococcus from the Oral Cavity by α-Amyrin Esters. Molecules. 2012; 17(11):12603-12611. https://doi.org/10.3390/molecules171112603
Chicago/Turabian StyleDíaz-Ruiz, Gloria, Liliana Hernández-Vázquez, Héctor Luna, María Del Carmen Wacher-Rodarte, and Arturo Navarro-Ocaña. 2012. "Growth Inhibition of Streptococcus from the Oral Cavity by α-Amyrin Esters" Molecules 17, no. 11: 12603-12611. https://doi.org/10.3390/molecules171112603




