Improved Antileishmanial Activity of Dppz through Complexation with Antimony(III) and Bismuth(III): Investigation of the Role of the Metal
Abstract
:1. Introduction
2. Results and Discussion
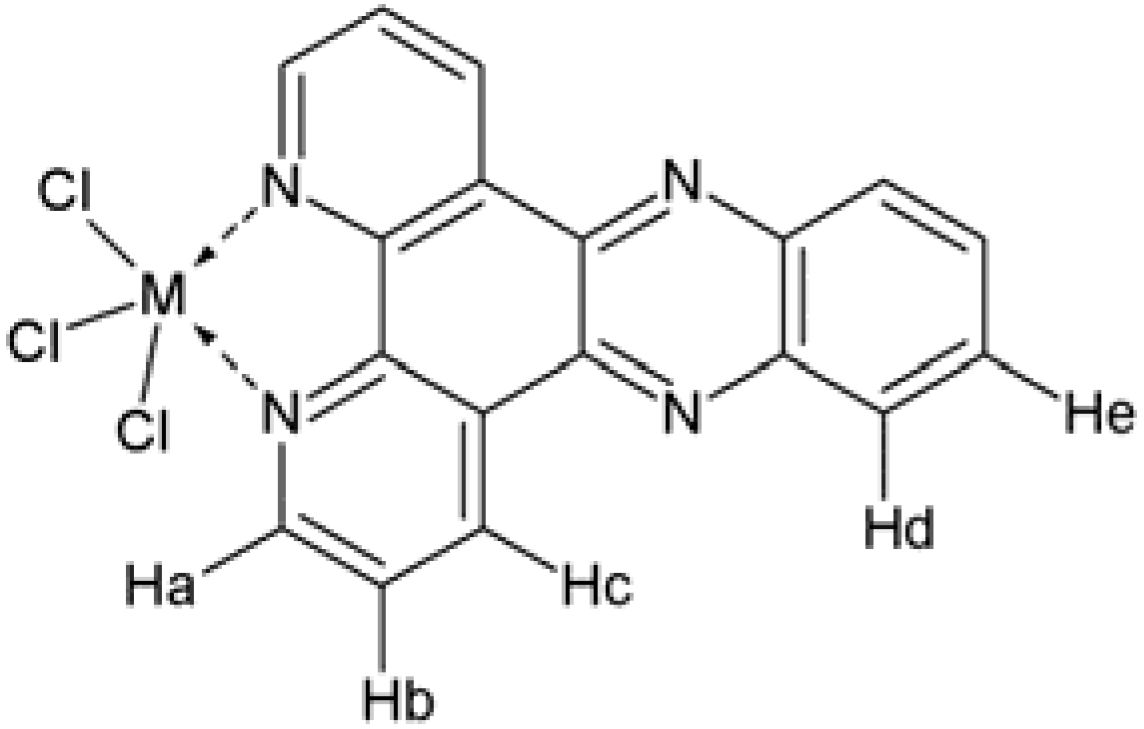
| Compound | 1H-NMR data (ppm) | References | ||||
|---|---|---|---|---|---|---|
| Hc | Ha | Hd | He | Hb | ||
| Dppz | 9.55 | 9.23 | 8.41 | 8.07 | 7.95 | Navarro et al. (2006) [26] |
| Sb(dppz)Cl3 | 9.68 | 9.31 | 8.39 | 8.18 | 8.11 | This work |
| Bi(dppz)Cl3 | 9.54 | 9.39 | 8.32 | 8.07 | 8.05 | |
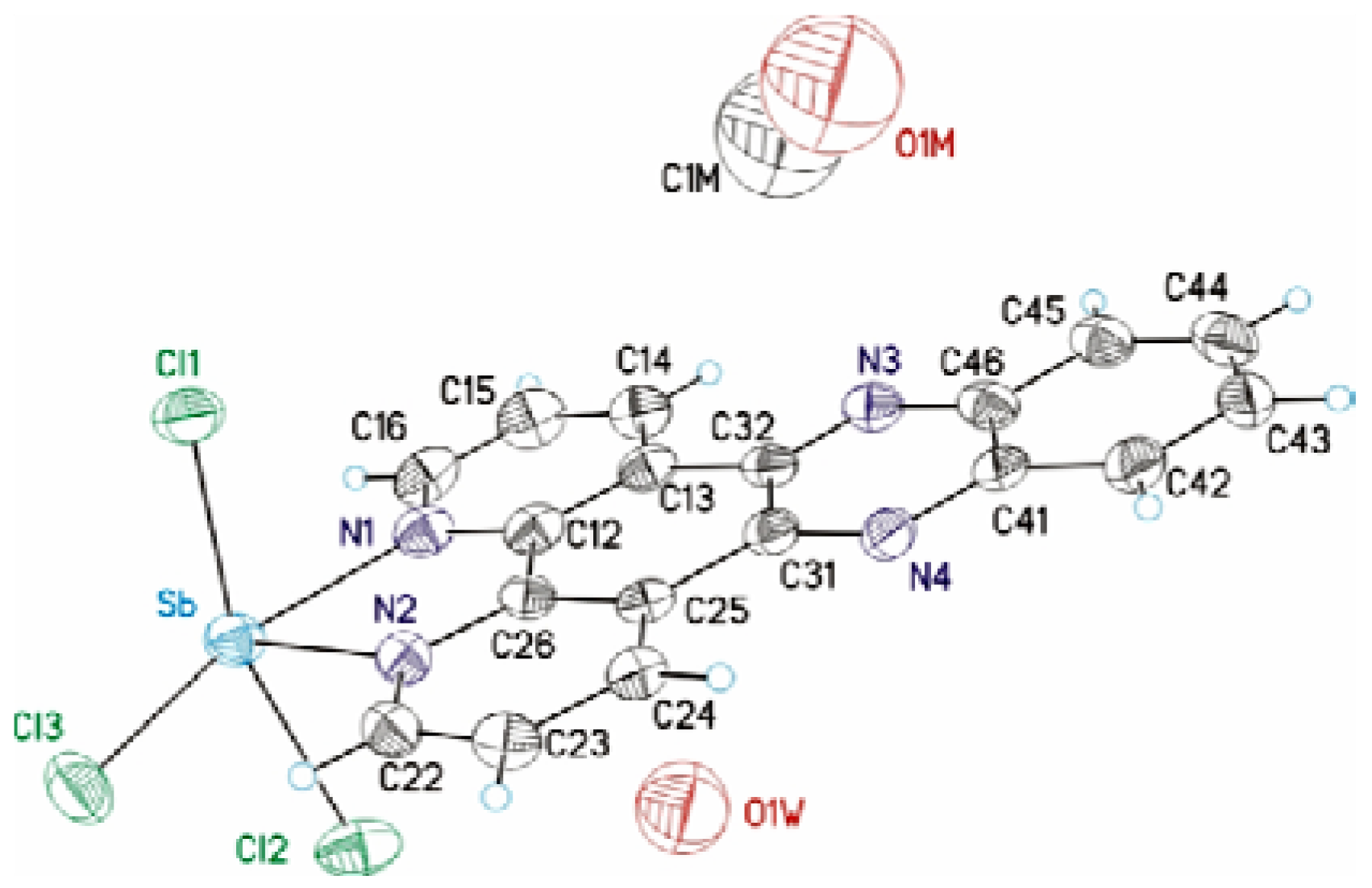
| Sb(dppz)Cl3 | |
|---|---|
| Empirical formula | C19H10Cl3 N4O1.25Sb |
| Formula weight | 542.41 |
| Temperature (K) | 293(2) |
| Crystal system | Monoclinic |
| Space group | P2(1)/c |
| a (Å) | 10.3244(2) |
| b (Å) | 13.2760(3) |
| c (Å) | 14.5753(3) |
| α (o) | 90 |
| β (o) | 92.0930(19) |
| γ (o) | 90 |
| V (Å3) | 1994.44(7) |
| Z | 4 |
| F (000) | 1056 |
| Dcalc (mg/m3) | 1.805 |
| Crystal dimensions mm3 | 0.2 × 0.2 × 0.3 |
| θ Range (o) | 4.29-62.65 |
| Reflections collected | 9035 |
| Independent reflection | 3138 |
| Rint | 0.0355 |
| Maximum/minimum transmission | 1.000 / 0.30303 |
| Data/restraints/parameters | 3138 / 0 / 247 |
| Goodness-of-fit on F2 | 1.060 |
| Final R indices | R1 = 0.0352, wR2 = 0.0826 |
| [I > 2σ(I)] | 2478 |
| R indices (all data) | R1 = 0.0522, wR2 = 0.0886 |
| Largest difference in peak/hole (e·A−3) | 0.761 and −0.560 |
| Sb-N(2) | 2.245(4) | N(2)-Sb-N(1) | 71.44(14) |
| Sb-N(1) | 2.345(4) | N(1)-Sb-Cl(3) | 159.07(10) |
| Sb-Cl(3) | 2.4992(15) | N(2)-Sb-Cl(3) | 87.73(11) |
| Sb-Cl(1) | 2.5126(13) | N(2)-Sb-Cl(1) | 82.14(10) |
| Sb-Cl(2) | 2.6348(14) | N(1)-Sb-Cl(1) | 84.23(10) |
| N(1)-C(16) | 1.334(6) | Cl(3)-Sb-Cl(1) | 95.22(5) |
| N(1)-C(12) | 1.351(6) | N(2)-Sb-Cl(2) | 80.25(10) |
| N(2)-C(22) | 1.336(6) | N(1)-Sb-Cl(2) | 82.97(10) |
| N(2)-C(26) | 1.368(6) | Cl(3)-Sb-Cl(2) | 91.68(5) |
| Cl(1)-Sb-Cl(2) | 160.80(5) |
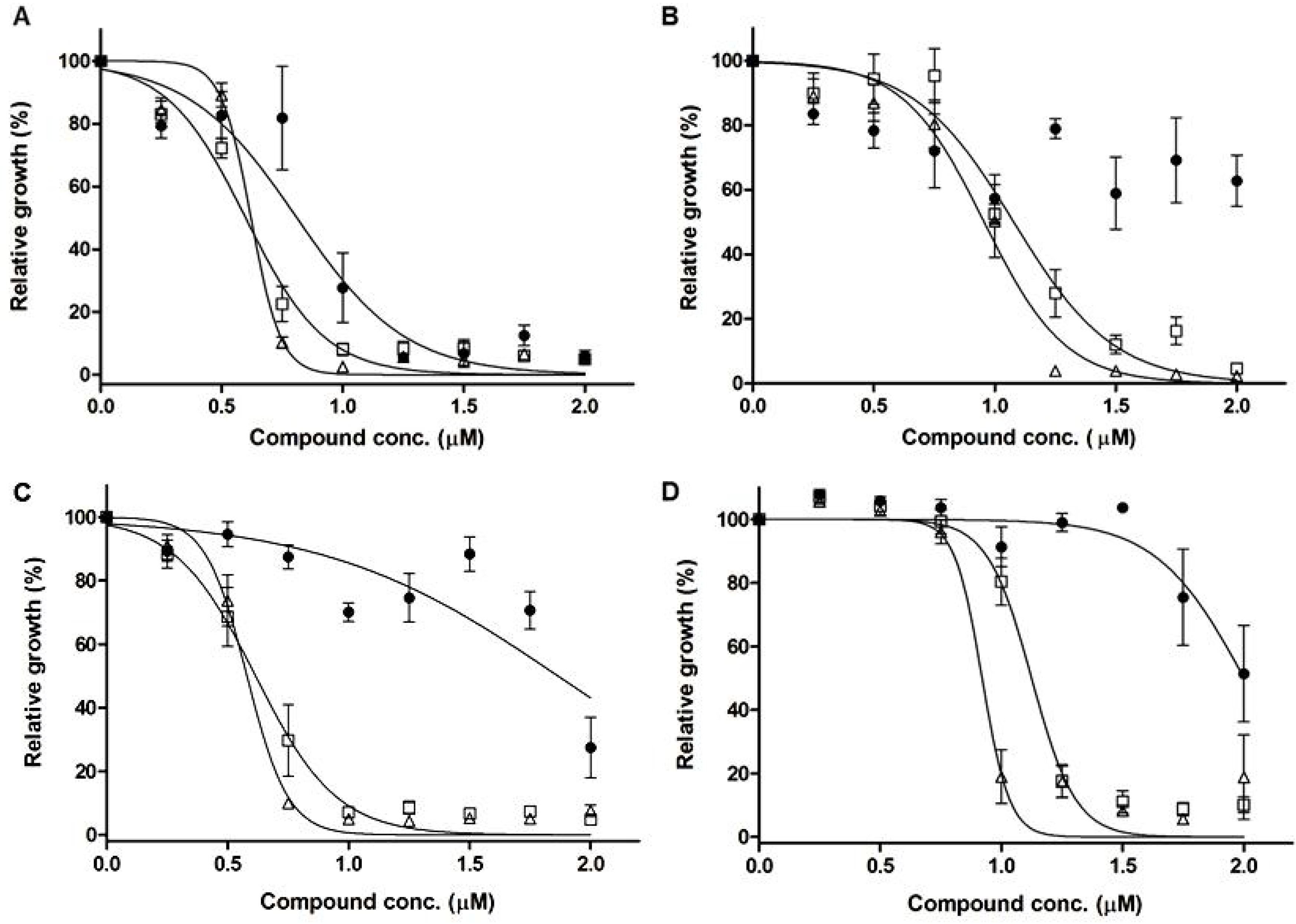
| Strain | IC50 (μM) ± SEM (CI 95%) | |||||
|---|---|---|---|---|---|---|
| Dppz | dppzBiCl3 | dppzSbCl3 | TA * | SbCl3 | BiCl3 | |
| L. infantum chagasi (WT) | 0.81 ± 0.04 | 0.59 ± 0.01 | 0.62 ± 0.01 | 100 ± 3 | 341 ± 2 | 563 ± 2 |
| (0.71–0.9) | (0.56–0.63) | (0.59–0.65) | ||||
| L. infantum chagasi (SbR) | 1.86 ± 0.08 | 0.61 ± 0.02 | 0.57 ± 0.01 | >2,700 | 462 ± 3 | >500 |
| (1.69–2.03) | (0.56–0.66) | (0.55–0.60) | ||||
| L. amazonensis (WT) | >2 | 1.07 ± 0.03 | 0.95 ± 0.02 | 83 ± 1 | 362 ± 2 | 621 ± 2 |
| (1.01–1.14) | (0.89–1.01) | |||||
| L. amazonensis (SbR) | 2.00 ± 0.05 | 1.12 ± 0.01 | 0.92 ± 0.02 | >2,700 | 1503 ± 2 | >500 |
| (1.89–2.10) | (1.08–1.16) | (0.86-0.97) | ||||
| Dppz | [Bi(dppz)Cl3] | [Sb(dppz)Cl3] | |
|---|---|---|---|
| CC50 (μM) * | 12.5 | 4.8 | 7.0 |
| SI # | 15.4 | 8.1 | 11.3 |
3. Experimental
3.1. General
3.2. Synthesis of the Complexes [Sb(dppz)Cl3] and [Bi(dppz)Cl3]
3.2.1. Synthesis of [Sb(dppz)Cl3] (1)
3.2.2. Synthesis of [Bi(dppz)Cl3] (2)
3.2.3. Stability of the Complexes
3.3. X-ray Crystallography
3.4. Antileishmanial and Cytotoxic Activities
3.4.1. Parasite Culture
3.4.2. Antileishmanial Activity Assay
3.4.3. Cytotoxicity Assay against Peritoneal Macrophages
3.5. Statistical Analysis
3.6. n-Octanol/Water Partition Coefficient
4. Conclusions
Acknowledgments
References
- Marsden, P.D. Pentavalent antimonials: Old drugs for new diseases. Rev. Soc. Bras. Med. Trop. 1985, 18, 187–198. [Google Scholar]
- Frézard, F.; Demicheli, C.; Ribeiro, R.R. Pentavalent antimonials: New perspectives for old drugs. Molecules 2009, 14, 2317–2336. [Google Scholar]
- Frézard, F.; Demicheli, C.; Ferreira, C.S.; Costa, M.A.P. Glutathione-induced conversion of pentavalent antimony to trivalent antimony in meglumine antimoniate. Antimicrob. Agents Chemother. 2001, 45, 913–916. [Google Scholar]
- Ferreira, C.D.; Martins, P.S.; Demicheli, C.; Brochu, C.; Ouellette, M.; Frézard, F. Thiol-induced reduction of antimony(V) into antimony(III): A comparative study with trypanothione, cysteinyl-glycine, cysteine and glutathion. Biometals 2003, 16, 441–446. [Google Scholar] [CrossRef]
- Sundar, S.; More, D.K.; Singh, M.K.; Singh, V.P.; Sharma, S.; Makharia, A.; Kumar, P.C.K.; Murray, H.W. Failure of pentavalent antimony in visceral leishmaniasis in India: Report from the center of the Indian epidemic. Clin. Infect. Dis. 2000, 31, 1104–1107. [Google Scholar]
- Ouellette, M.; Légaré, D.; Haimeur, A.; Grondin, K.; Roy, G.; Brochu, C.; Papadopoulou, B. ABC transporters in Leishmania and their role in drug resistance. Drug Resist. Updat. 1998, 1, 43–48. [Google Scholar] [CrossRef]
- Légaré, D.; Richard, D.; Mukhopadhyay, R.; Stierhof, Y.D.; Rosen, B.P.; Haimeur, A.; Papadopoulou, B.; Ouellette, M. The Leishmania ATP-binding cassette protein PGPA is an intracellular metal-thiol transporter ATPase. J. Biol. Chem. 2001, 276, 26301–26307. [Google Scholar]
- Shaked-Mishan, P.; Ulrich, N.; Ephros, M.; Zilberstein, D. Novel intracellular SbV reducing activity correlates with antimony susceptibility in Leishmania donovani. J. Biol. Chem. 2001, 276, 3971–3976. [Google Scholar]
- Gourbal, B.; Sonuc, N.; Bhattacharjee, H.; Légaré, D.; Sundar, S.; Ouellette, M.; Rosen, B.P.; Mukhopadhyay, R. Drug uptake and modulation of drug resistance in Leishmania by an aquaglyceroporin. J. Biol. Chem. 2004, 279, 31010–31017. [Google Scholar]
- Ayres, D.C.; Pinto, L.A.; Giorgio, S. Efficacy of pentavalent antimony, amphotericin B, and miltefosine in Leishmania amazonensis-infected macrophages under normoxic and hypoxic conditions. J. Parasitol. 2008, 94, 1415–1417. [Google Scholar] [CrossRef]
- Silvestru, C.; Socaciu, C.; Bara, A.; Haiduc, I. The first organoantimony(III) compounds possessing antitumor properties: Diphenylantimony(III) derivatives of dithiophosphorus ligand. Anticancer Res. 1990, 10, 803–804. [Google Scholar]
- Sereno, D.; Holzmuller, P.; Mangot, I.; Cuny, G.; Ouaissi, A.; Lemesre, J.L. Antimonial-mediated DNA fragmentation in Leishmania infantum amastigotes. Antimicrob. Agents Chemother. 2001, 45, 2064–2069. [Google Scholar] [CrossRef]
- Moreira, W.; Leprohon, P.; Ouellette, M. Tolerance to drug-induced cell death favours the acquisition of multidrug resistance in Leishmania. Cell Death Dis. 2011, 2, 1–8. [Google Scholar]
- Wyllie, S.; Fairlamb, A.H. Differential toxicity of antimonial compounds and their effects on glutathione homeostasis in a human leukaemia monocyte cell line. Biochem. Pharmacol. 2006, 71, 257–267. [Google Scholar]
- Cunningham, M.L.; Fairlamb, A.H. Trypanothione reductase from Leishmania donovani Purification, characterization and inhibition by trivalent antimonials. Eur. J. Biochem. 1995, 230, 460–468. [Google Scholar] [CrossRef]
- Baiocco, P.; Colotti, G.; Franceschini, S.; Ilari, A. Molecular basis of antimony treatment in leishmaniasis. J. Med. Chem. 2009, 52, 2603–2612. [Google Scholar]
- Klapotke, T. Biological activity of organometallic bismuth compounds. Biol. Met. 1988, 1, 69–76. [Google Scholar]
- Yang, N.; Sun, H. Biocoordination chemistry of bismuth: Recent advances Coord. Chem. Rev. 2007, 251, 2354–2366. [Google Scholar]
- Andrews, P.C.; Frank, R.; Junk, P.C.; Kedzierski, L.; Kumar, I.; MacLellan, J.G. Anti-Leishmanial activity of homo- and heteroleptic bismuth(III) carboxylates. J. Inorg. Biochem. 2011, 105, 454–461. [Google Scholar]
- Ensafi, A.A.; Hajian, R.; Ebrahimi, S. Study on the interaction between Morin-Bi(III) complex and DNA with the use of methylene blue dye as a fluorophor probe. J. Braz. Chem. Soc. 2009, 20, 266–276. [Google Scholar] [CrossRef]
- Oliveira, L.G.; Silva, M.M.; de Paula, F.C.S.; Pereira-Maia, E.C.; Donnici, C.L.; de Simone, C.A.; Frézard, F.; da Silva Junior, E.N.; Demicheli, C. Antimony(V) and bismuth(V) complexes of lapachol: Synthesis, crystal structure and cytotoxic activity. Molecules 2011, 16, 10314–10323. [Google Scholar] [CrossRef]
- Sasmal, P.K.; Patra, A.K.; Nethaji, M.; Chakravarty, A.R. DNA cleavage by new oxovanadium(IV) complexes of N-salicylidene α-amino acids and phenanthroline bases in the photodynamic therapy window. Inorg. Chem. 2007, 46, 11112–11121. [Google Scholar]
- Toneatto, J.; Boero, R.A.; Lorenzatti, G.; Cabanillas, A.M.; Argueello, G.A. New insights in the DNA-[Cr(phen)2(dppz)]3+ binding and photocleavage properties by the complex with an intercalating ligand. J. Inorg. Biochem. 2010, 104, 697–703. [Google Scholar]
- Friedman, A.E.; Chambron, J.C.; Sauvage, J.P.; Turro, N.J.; Barton, J.K. Molecular light switch for DNA: Ru(bpy)2(dppz)2+. J. Am. Chem. Soc. 1990, 112, 4960–4962. [Google Scholar] [CrossRef]
- Zhang, K.Y.; Li, S.P.Y.; Zhu, N.; Or, I.W.S.; Cheung, M.S.H.; Lam, Y.W.; Lo, K.K.W. Structure, photophysical and electrochemical properties, biomolecular interactions, and intracellular uptake of luminescent cyclometalated iridium(III) dipyridoquinoxaline complexes. Inorg. Chem. 2010, 49, 2530–2540. [Google Scholar] [CrossRef]
- Navarro, M.; Hernandez, C.; Colmenares, I.; Hernandez, P.; Fernandez, M.; Sierralta, A.; Marchan, E. Synthesis and characterization of [Au(dppz)2]Cl3: DNA Interaction studies and biological activity against Leishmania (L) mexicana. J. Inorg. Biochem. 2007, 101, 111–116. [Google Scholar] [CrossRef]
- Benítez, J.; Guggeri, L.; Tomaz, I.; Pessoa, J.C.; Moreno, V.; Lorenzo, J.; Avilés, F.X.; Garat, B.; Gambino, D. A novel vanadyl complex with a polypyridyl DNA intercalator as ligand: A potential anti-protozoa and anti-tumor agent. J. Inorg. Biochem. 2009, 103, 1386–1387. [Google Scholar]
- Yin, H.D.; Zhai, J. Synthesis, characterizations and crystal structures of antimony(III) complexes with nitrogen-containing ligands. Inorg. Chim. Acta 2009, 362, 339–345. [Google Scholar] [CrossRef]
- Espínola, J.G.P.; Martins, E.P.S.; Aguiar, F.P.; Silva, H.R.M.; Fonseca, M.G.; Arakaki, L.N.H.; Teotôno, E.E.S. Thermal decomposition study of bismuth (III) trichloride complex with 1,10-phenanthroline as the ligand. J. Therm. Anal. Calorim. 2011, 106, 601–606. [Google Scholar]
- Bertazzi, N.; Alonzo, G.; Gibb, T.C. Antimony-121 moessbauer and infrared spectral studies on 1,10-phenanthroline adducts of antimony(III) halides. Inorg. Chim. Acta 1983, 73, 121–124. [Google Scholar] [CrossRef]
- Shaw, J.J. Further thoughts on the use of the name Leishmania (Leishmania) infantum chagasi for the a etiological agent of American visceral leishmaniasis. Mem. Inst. Oswaldo Cruz 2006, 101, 577–579. [Google Scholar] [CrossRef]
- Marzochi, M.C.D.A.; Marzochi, K.B.F. Tegumentary and visceral leishmaniasis in Brazil emerging anthropozoonosis and possibilities for their control. Cad. Saúde Públ. 1994, 10, 359–375. [Google Scholar] [CrossRef]
- Sinagra, A.; Luna, C.; Abraham, D.; Iannella, M.D.C.; Riarte, A.; Krolewiecki, A.J. The activity of azithromycin against Leishmania (Viannia) braziliensis and Leishmania (Leishmania) amazonensis in the golden hamster model. Rev. Soc. Bras. Med. Trop. 2007, 40, 627–630. [Google Scholar] [CrossRef]
- Navarro, M.; Cisneros-Fajardo, E.J.; Sierralta, A.; Fernandez-Mestre, M.; Silva, P.; Arrieche, D.; Marchán, E. Design of copper DNA intercalators with leishmanicidal activity. J. Biol. Inorg. Chem. 2003, 8, 401–408. [Google Scholar]
- Pramanik, N.C.; Pramanik, K.; Ghosh, P.; Bhattacharya, S. Chemistry of [Ru(tpy)(pap)(L')n+ (tpy = 2,2',6',2 ''-terpyridine; pap = 2-(phenylazo)pyridine; L' = Cl−, H2O, CH3CN, 4-picoline, N3−; n = 1,2. X-ray crystal structure of [Ru(tpy)(pap)(CH3CN)](ClO4)2 and catalytic oxidation of water to dioxygen by [Ru(tpy)(pap)(H2O)]2+. Polyhedron 1998, 17, 1525–1534. [Google Scholar] [CrossRef]
- Ossipov, D.; Gohil, S.; Chattopadhyaya, J. Synthesis of the DNA-[Ru(tpy)(dppz)(CH3CN)]2+ conjugates and their photo cross-linking studies with the complementary DNA strand. J. Am. Chem. Soc. 2002, 124, 13416–13433. [Google Scholar]
- Liang, B.; Zhu, M.X.; Zhu, W.G. Synthesis and Photoluminescence of New Europium Complex Eu(DBM)3(DPPZ) with Dipyridophenazine Ligand. Chin. Chem. Lett. 2003, 14, 43–46. [Google Scholar]
- Sheldrick, G.M.S. SHELXL-97—A Program for Crystal Structure Refinement; University of Goettingen: Goettingen, Germany, 1997. [Google Scholar]
- Ouellette, M.; Borst, P. Drug-resistance and P-glycoprotein gene amplification in the protozoan parasite Leishmania. Res. Microbiol. 1991, 142, 737–746. [Google Scholar] [CrossRef]
- Monte-Neto, R.L.; Coelho, A.C.; Raymond, F.; Légaré, D.; Corbeil, J.; Melo, M.N.; Frézard, F.; Ouellette, M. Gene Expression Profiling and Molecular Characterization of Antimony Resistance in Leishmania amazonensis. PLoS Negl. Trop. Dis. 2011, 5, e1167. [Google Scholar]
- Ma, G.Y.; Khan, S.I.; Jacob, M.R.; Tekwani, B.L.; Li, Z.Q.; Pasco, D.S.; Walker, L.A.; Khan, L.A. Antimicrobial and antileishmanial activities of hypocrellins A and B. Antimicrob. Agents Chemother. 2004, 48, 4450–4452. [Google Scholar]
- Habtemariam, S. In vitro antileishmanial effects of antibacterial diterpenes from two Ethiopian Premma species: P. chimperi and P. oligotricha. BMC Pharmacol. 2003, 3, 1–6. [Google Scholar] [CrossRef]
- Ouellette, M.; Fase-Fowler, F.; Borst, P. The amplified H circle of methotrexate-resistant leishmania tarentolae contains a novel P-glycoprotein gene. EMBO J. 1990, 9, 1027–1033. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Wilson, J.J.; Lippard, S.J. In Vitro Anticancer Activity of cis-Diammineplatinum(II) Complexes with β-Diketonate Leaving Group Ligands. J. Med. Chem. 2012, 55, 5326–5336. [Google Scholar]
- Sample Availability: Samples of the antimony(III) and bismuth(III) complexes of dppz are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lizarazo-Jaimes, E.H.; Monte-Neto, R.L.; Reis, P.G.; Fernandes, N.G.; Speziali, N.L.; Melo, M.N.; Frézard, F.; Demicheli, C. Improved Antileishmanial Activity of Dppz through Complexation with Antimony(III) and Bismuth(III): Investigation of the Role of the Metal. Molecules 2012, 17, 12622-12635. https://doi.org/10.3390/molecules171112622
Lizarazo-Jaimes EH, Monte-Neto RL, Reis PG, Fernandes NG, Speziali NL, Melo MN, Frézard F, Demicheli C. Improved Antileishmanial Activity of Dppz through Complexation with Antimony(III) and Bismuth(III): Investigation of the Role of the Metal. Molecules. 2012; 17(11):12622-12635. https://doi.org/10.3390/molecules171112622
Chicago/Turabian StyleLizarazo-Jaimes, Edgar H., Rubens L. Monte-Neto, Priscila G. Reis, Nelson G. Fernandes, Nivaldo L. Speziali, Maria N. Melo, Frédéric Frézard, and Cynthia Demicheli. 2012. "Improved Antileishmanial Activity of Dppz through Complexation with Antimony(III) and Bismuth(III): Investigation of the Role of the Metal" Molecules 17, no. 11: 12622-12635. https://doi.org/10.3390/molecules171112622





