Bis-sulfonic Acid Ionic Liquids for the Conversion of Fructose to 5-Hydroxymethyl-2-furfural
Abstract
:1. Introduction
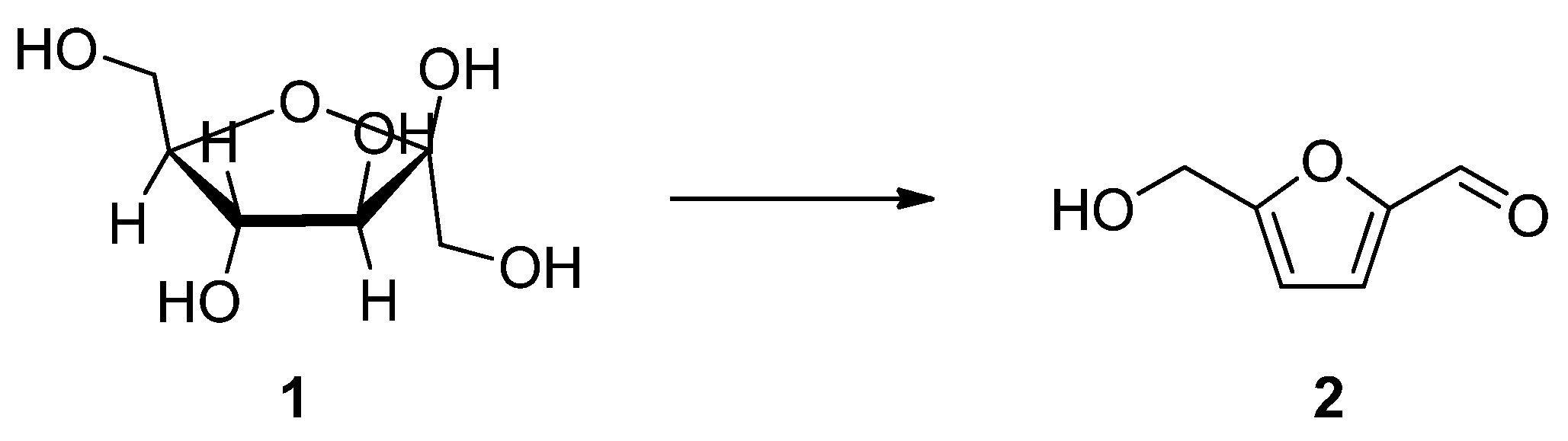
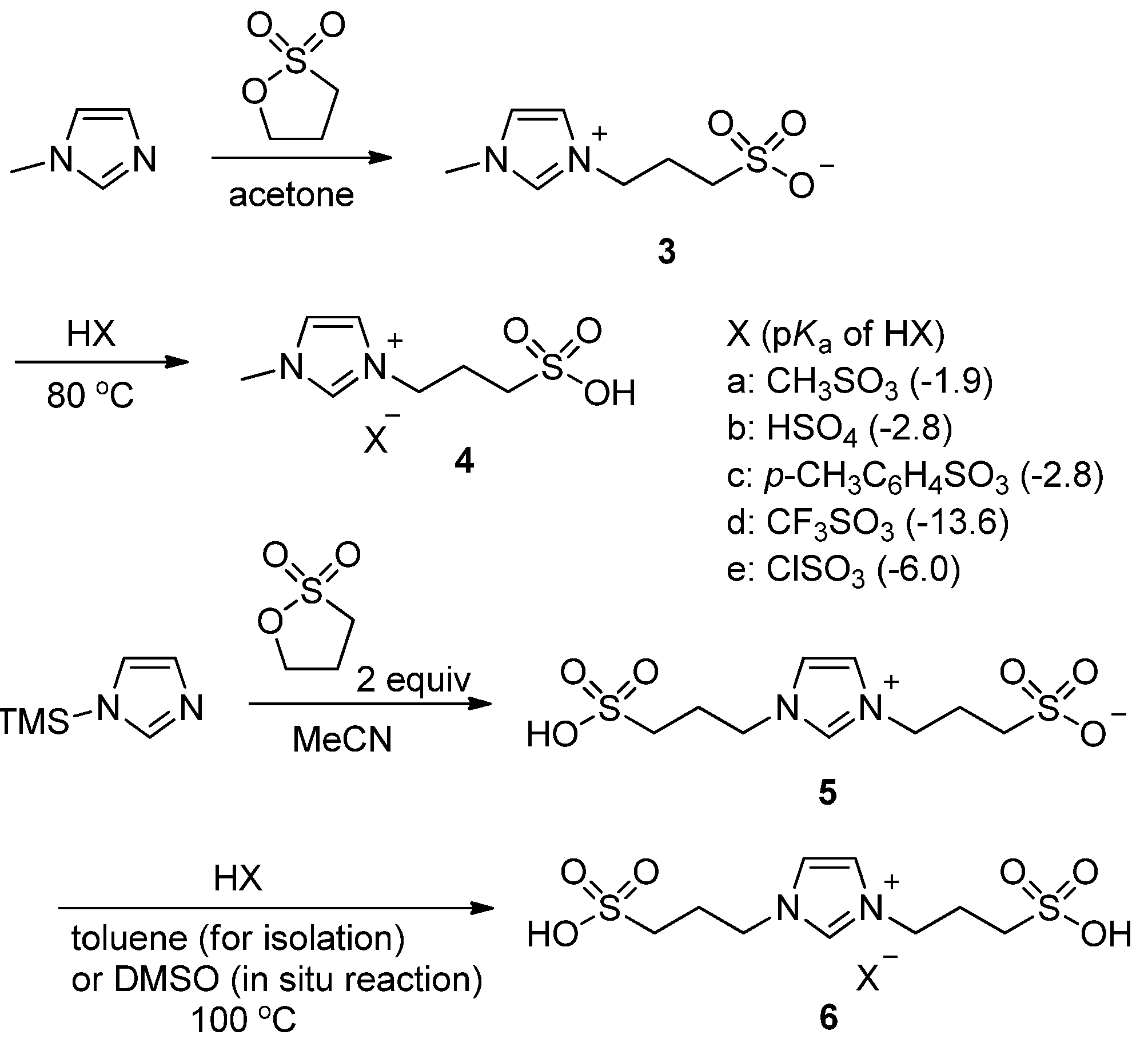
2. Results and Discussion
| Entry | 3 (equiv.) | MsOH (equiv.) | Solvent (mL) | Conversion (%) b | Yield 2 (%) c | Selectivity (%) |
|---|---|---|---|---|---|---|
| 1 | - | 1 | EtOAc (10) | 96.3 | 5.0 | 5.2 |
| 2 | - | 10 | - | 81.5 | 3.0 | 3.7 |
| 3 | 1 | 1 | - | 99.5 | 5.0 | 5.0 |
| 4 | 10 | 10 | - | 95.6 | 30.8 | 32.2 |
| 5 | 1 | 1 | EtOAc (10) | 99.6 | 41.0 | 41.2 |
| 6 | 1 | 1 | H2O (10) | 17 | 0.7 | 4.1 |
| 7 | 1 | 1 | MeCN (10) | 92.7 | 41.3 | 44.6 |
| 8 | 1 | 1 | DMSO (10) | 100 | 59.8 | 59.8 |
| 9 | 1 | 1 | DMSO (20) | 100 | 59.3 | 59.3 |
| 10 | 1 | 1 | DMSO (2) | 100 | 65.6 | 65.6 |
| Entry | Ionic liquid (equiv.) | Temp. (°C) | Time (h) | Yield 2 (%) b |
|---|---|---|---|---|
| 1 | - | 100 | 1 | 15 |
| 2 | 4a (0.1) | 100 | 1 | 48 |
| 3 | 4a (0.3) | 100 | 1 | 51 |
| 4 | 4a (0.5) | 100 | 1 | 59 |
| 5 | 4a (0.7) | 100 | 1 | 62 |
| 6 | 4a (1.0) | 100 | 1 | 66 |
| 7 | 4a (2.0) | 100 | 1 | 50 |
| 8 | 4a (1.0) | 80 | 1 | 54 |
| 9 | 4a (1.0) | 120 | 1 | 54 |
| 10 | 4a (1.0) | 100 | 0.5 | 52 |
| 11 | 4a (1.0) | 100 | 2 | 66 |
| 12 | 4b (1.0) | 100 | 1 | 65 |
| 13 | 4c (1.0) | 100 | 1 | 67 |
| 14 | 4d (1.0) | 100 | 1 | 63 |
| 15 | 4e (1.0) | 100 | 1 | 23 |
| 16 | 5 (1.0) | 100 | 1 | 57 |
| 17 | 6a (1.0) | 100 | 1 | 75 |
| 18 | 6b (1.0) | 100 | 1 | 72 |
| 19 | 6c (1.0) | 100 | 1 | 64 |
| 20 | 6d (1.0) | 100 | 1 | 55 |
| 21 | 6e (1.0) | 100 | 1 | 33 |
3. Experimental
3.1. General
3.2. Preparation of 3
3.3. Representative Procedure for the Preparation of 4a
3.4. Preparation of 5
3.5. Representative Procedure for the Preparation of 6a
3.6. Representative Procedure for 5-HMF (2) Using the Acid Ionic Liquid 6a
4. Conclusions
Acknowledgments
- Sample Availability: Samples of the compounds 5 and 6 are available from the authors
References
- Ragauskas, A.J.; Williams, C.K.; Davison, B.H.; Britovsek, G.; Cairney, J.; Eckert, C.A.; Frederick, W.J., Jr; Hallett, J.P.; Leak, D.J.; Liotta, C.L.; et al. The path forward for biofuels and biomaterials. Science 2006, 311, 484–489. [Google Scholar]
- Roman-Leshkov, Y.; Chheda, J.N.; Dumesic, J.A. Phase modifiers promote efficient production of hydroxymethylfurfural from fructose. Science 2006, 312, 1933–1937. [Google Scholar] [CrossRef]
- Roman-Leshkov, Y.; Barrett, C.J.; Liu, Z.Y.; Dumesic, J.A. Production of dimethylfuran for liquid fuels from biomass-derived carbohydrates. Nature 2007, 447, 982–986. [Google Scholar]
- Lichtenthaler, F.W. Unsaturated O- and N-heterocycles from carbohydrate feedstocks. Acc. Chem. Res. 2002, 35, 728–737. [Google Scholar]
- Binder, J.B.; Raines, R.T. Simple chemical transformation of lignocellulosic biomass into furans for fuels and chemicals. J. Am. Chem. Soc. 2009, 131, 1979–1985. [Google Scholar]
- Zhao, H.; Holladay, J.E.; Brown, H.; Zhang, Z.C. Metal chlorides in ionic liquid solvents convert sugars to 5-hydroxymethylfurfural. Science 2007, 316, 1597–1600. [Google Scholar] [CrossRef]
- Yong, G.; Zhang, Y.; Ying, J.Y. Efficient catalytic system for the selective production of 5-hydroxymethylfurfural from glucose and fructose. Angew. Chem. Int. Ed. 2008, 47, 9345–9348. [Google Scholar] [CrossRef]
- Sievers, C.; Musin, I.; Marzialetti, T.; Olarte, M.B.V.; Agrawal, P.K.; Jones, C.W. Acid-catalyzed conversion of sugars and furfurals in an ionic-liquid phase. ChemSusChem 2009, 2, 665–671. [Google Scholar] [CrossRef]
- Stahlberg, T.; Sorensen, M.G.; Riisager, A. Direct conversion of glucose to 5-(hydroxymethyl)furfural in ionic liquids with lanthanide catalysts. Green Chem. 2010, 12, 321–325. [Google Scholar] [CrossRef]
- Takagaki, A.; Ohara, M.; Nishimura, S.; Ebitani, K. A one-pot reaction for biorefinery: Combination of solid acid and base catalysts for direct production of 5-hydroxymethylfurfural from saccharides. Chem. Commun. 2009, 6276–6278. [Google Scholar]
- Huang, R.; Qi, W.; Su, R.; He, Z. Integrating enzymatic and acid catalysis to convert glucose into 5-hydroxymethylfurfural. Chem. Commun. 2010, 46, 1115–1117. [Google Scholar]
- Hu, S.; Zhang, Z.; Song, J.; Zhou, Y.; Han, B. Efficient conversion of glucose into 5-hydroxymethylfurfural catalyzed by a common Lewis acid SnCl4 in an ionic liquid. Green Chem. 2009, 11, 1746–1749. [Google Scholar] [CrossRef]
- Tong, X.; Li, Y. Efficient and selective dehydration of fructose to 5-hydroxymethylfurfural catalyzed by Brønsted-acidic ionic liquids. ChemSusChem 2010, 3, 350–355. [Google Scholar] [CrossRef]
- Tong, X.; Ma, Y.; Li, Y. An efficient catalytic dehydration of fructose and sucrose to 5-hydroxymethylfurfural with protic ionic liquids. Carbohydr. Res. 2010, 345, 1698–1701. [Google Scholar] [CrossRef]
- Fei, Z.; Zhao, D.; Geldbach, T.J.; Scopelliti, R.; Dyson, P.J. Brønsted acid ionic liquid and their zwitterions: Synthesis, characterization and pKa determination. Chem. Eur. J. 2004, 10, 4886–4893. [Google Scholar]
- Bao, Q.; Qiao, K.; Tomida, D.; Yokoyama, C. Preparation of 5-hydroxymethylfurfural by dehydration of fructose in the presence of acidic ionic liquid. Catal. Commun. 2008, 9, 1383–1388. [Google Scholar] [CrossRef]
- Jiang, F.; Zhu, Q.; Ma, D.; Liu, X.; Han, X. Direct conversion and NMR observation of cellulose to glucose and 5-hydroxymethylfurfural (HMF) catalyzed by the acidic ionic liquids. J. Mol. Catal. A 2011, 334, 8–12. [Google Scholar] [CrossRef]
- Tao, F.; Song, H.; Chou, L. Efficient conversion of cellulose into furans catalyzed by metal ions in ionic liquids. J. Mol. Catal. A 2012, 357, 11–18. [Google Scholar] [CrossRef]
- Tao, F.; Song, H.; Chou, L. Catalytic conversion of cellulose to chemicals in ionic liquid. Carbohydr. Res. 2011, 346, 58–63. [Google Scholar] [CrossRef]
- Tao, F.; Song, H.; Chou, L. Hydrolysis of cellulose in SO3H-functionalized ionic liquids. Bioresour. Technol. 2011, 102, 9000–9006. [Google Scholar] [CrossRef]
- Liu, X.; Ma, H.; Wu, Y.; Wang, C.; Yang, M.; Yan, P.; Welz-Biermann, U. Esterification of glycerol with acetic acid using double SO3H-functionalized ionic liquids as recoverable catalysts. Green Chem. 2011, 13, 697–701. [Google Scholar] [CrossRef]
- Xu, D.-Q.; Wu, J.; Luo, S.-P.; Zhang, J.-X.; Wu, J.-Y.; Du, X.-H.; Xu, Z.-Y. Fischer indole synthesis catalyzed by novel SO3H-functionalized ionic liquids in water. Green Chem. 2009, 11, 1239–1246. [Google Scholar] [CrossRef]
- Qi, X.; Watanabe, M.; Aida, T.M.; Smith, R.L., Jr. Catalytic dehydration of fructose into 5-hydroxymethylfurfural by ion-exchange resin in mixed-aqueous system by microwave heating. Green Chem. 2008, 10, 799–805. [Google Scholar] [CrossRef]
- Hu, S.; Zhang, Z.; Zhou, Y.; Han, B.; Fan, H.; Li, W.; Song, J.; Xie, Y. Conversion of fructose to 5-hydroxymethylfurfural using ionic liquids prepared from renewable materials. Green Chem. 2008, 10, 1280–1283. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sim, S.E.; Kwon, S.; Koo, S. Bis-sulfonic Acid Ionic Liquids for the Conversion of Fructose to 5-Hydroxymethyl-2-furfural. Molecules 2012, 17, 12804-12811. https://doi.org/10.3390/molecules171112804
Sim SE, Kwon S, Koo S. Bis-sulfonic Acid Ionic Liquids for the Conversion of Fructose to 5-Hydroxymethyl-2-furfural. Molecules. 2012; 17(11):12804-12811. https://doi.org/10.3390/molecules171112804
Chicago/Turabian StyleSim, Sang Eun, Sunjeong Kwon, and Sangho Koo. 2012. "Bis-sulfonic Acid Ionic Liquids for the Conversion of Fructose to 5-Hydroxymethyl-2-furfural" Molecules 17, no. 11: 12804-12811. https://doi.org/10.3390/molecules171112804




