Purification and Identification of Antioxidant Peptides from Enzymatic Hydrolysates of Tilapia (Oreochromis niloticus) Frame Protein
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation of Tilapia Frame Protein Hydrolysates (TFPH)
| Enzyme | DH | Antioxidant activities | |||
|---|---|---|---|---|---|
| DPPH | •O2 | H2O2 | •OH | ||
| Properase E | 13.8 ± 1.2 b | 50.8 ± 2.6 b | 42.6 ± 3.1 b | 70.0 ± 2.7 a | 84.5 ± 5.1 a |
| Pepsin | 5.3 ± 0.3 d | 26.0 ± 1.5 e | 12.9 ± 0.6 d | 28.6 ± 0.6 e | 23.7 ± 1.5 d |
| Trypsin | 15.1 ± 0.9 a | 70.1 ± 4.2 a | 58.5 ± 2.8 a | 72.2 ± 3.8 a | 89.0 ± 4.1 a |
| Flavourzyme | 12.7 ± 0.5 b | 54.4 ± 3.5 b | 40.4 ± 3.2 b | 59.4 ± 4.1 b | 68.9 ± 3.1 b |
| Neutrase | 8.6 ± 0.4 c | 33.8 ± 1.6 d | 24.3 ± 1.8 c | 41.5 ± 1.1 d | 57.2 ± 2.5 c |
| GC106 | 3.8 ± 0.1 e | 26.7 ± 0.9 e | 11.5 ± 0.7 d | 29.1 ± 1.4 e | 24.3 ± 0.9 d |
| Papain | 9.5 ± 0.8 c | 41.5 ± 2.1 c | 26.1 ± 1.6 c | 50.7 ± 2.8 c | 59.6 ± 1.6 c |
2.2. Ultrafiltration
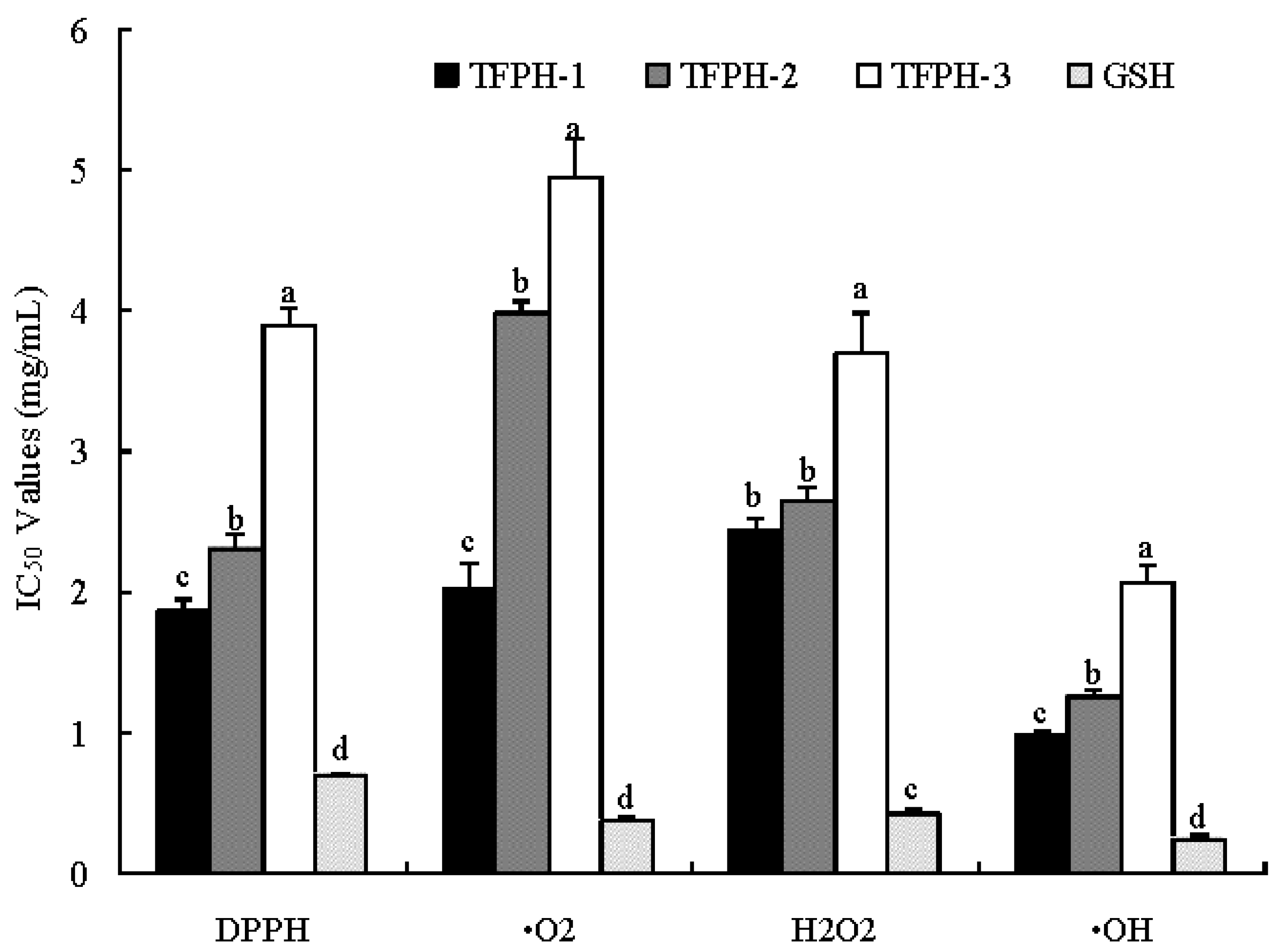
2.3. Purification of Antioxidant Peptides
2.3.1. Purification of Antioxidant Peptide Using Ion-Exchange Chromatography
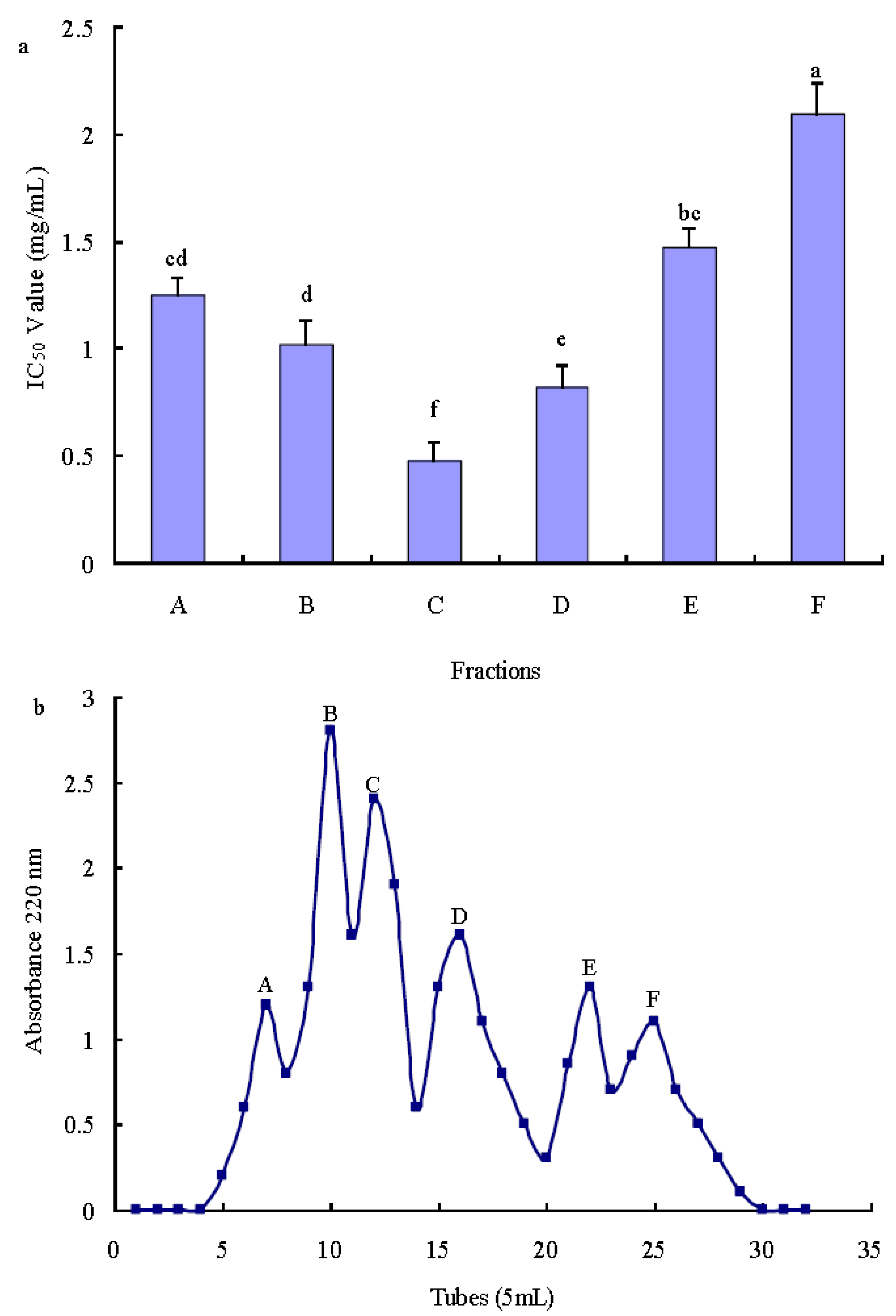
2.3.2. Purification of Antioxidant Peptide Using Gel Filtration
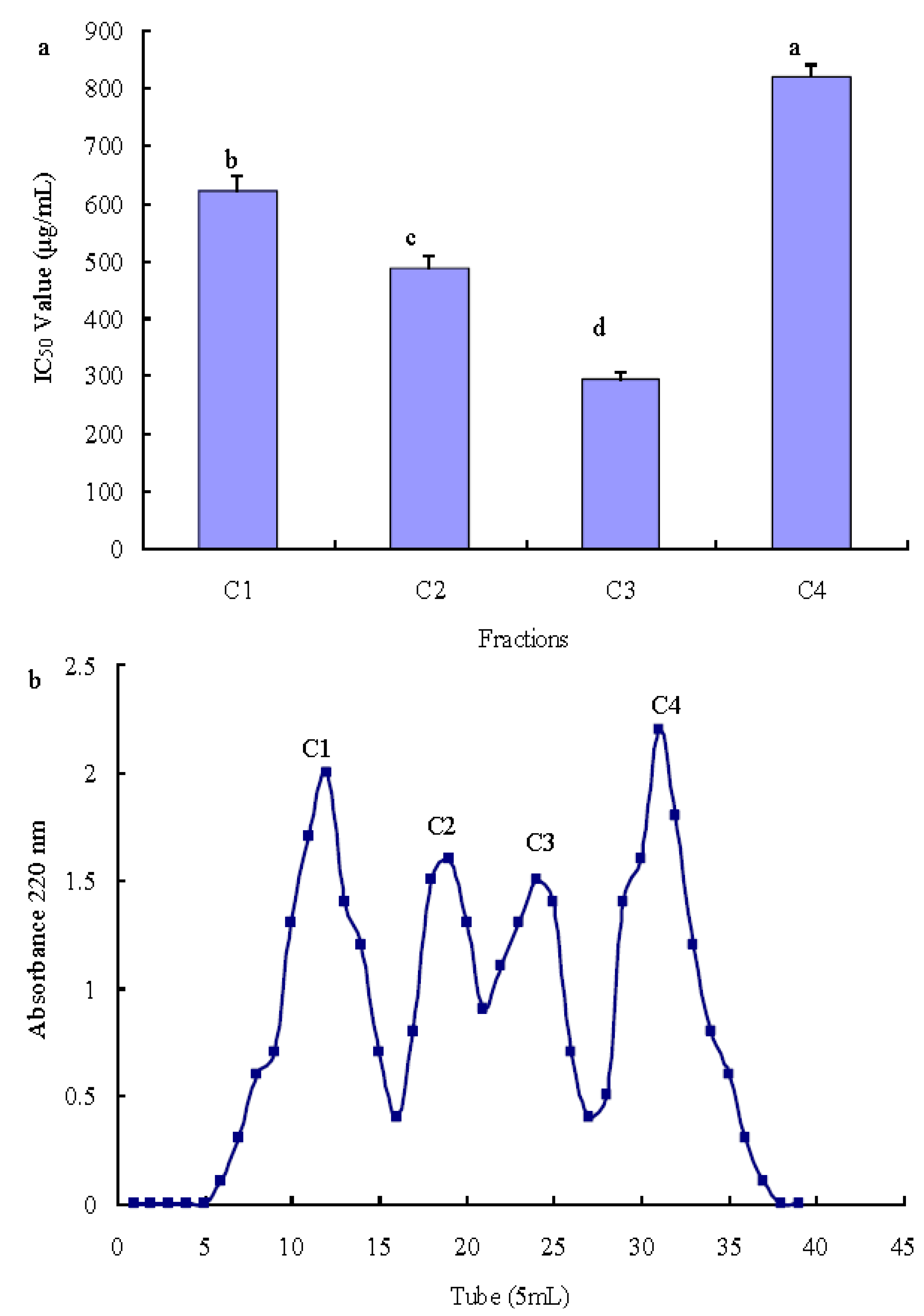
2.3.3. Purification of Antioxidant Peptide Using RP-HPLC
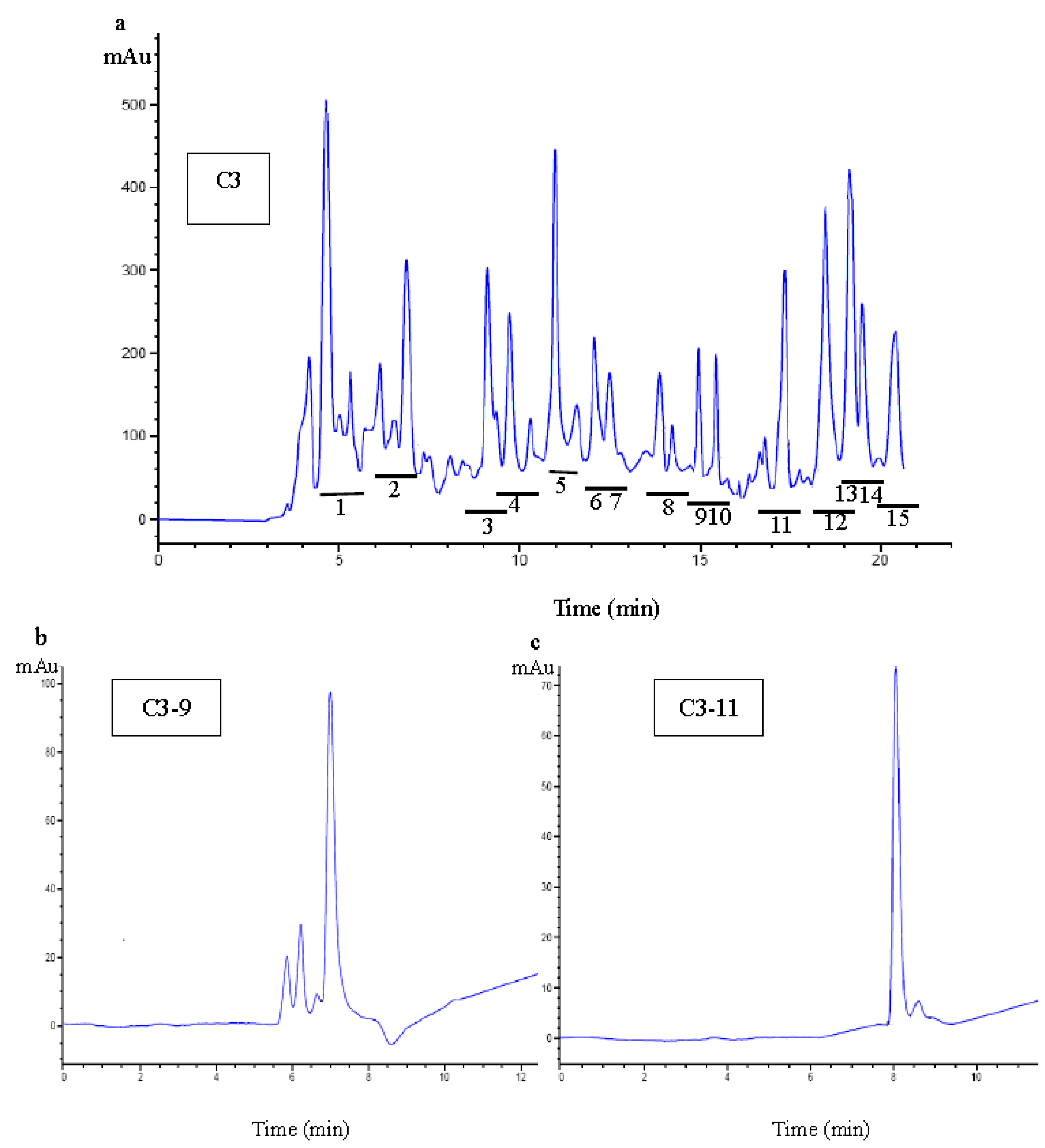
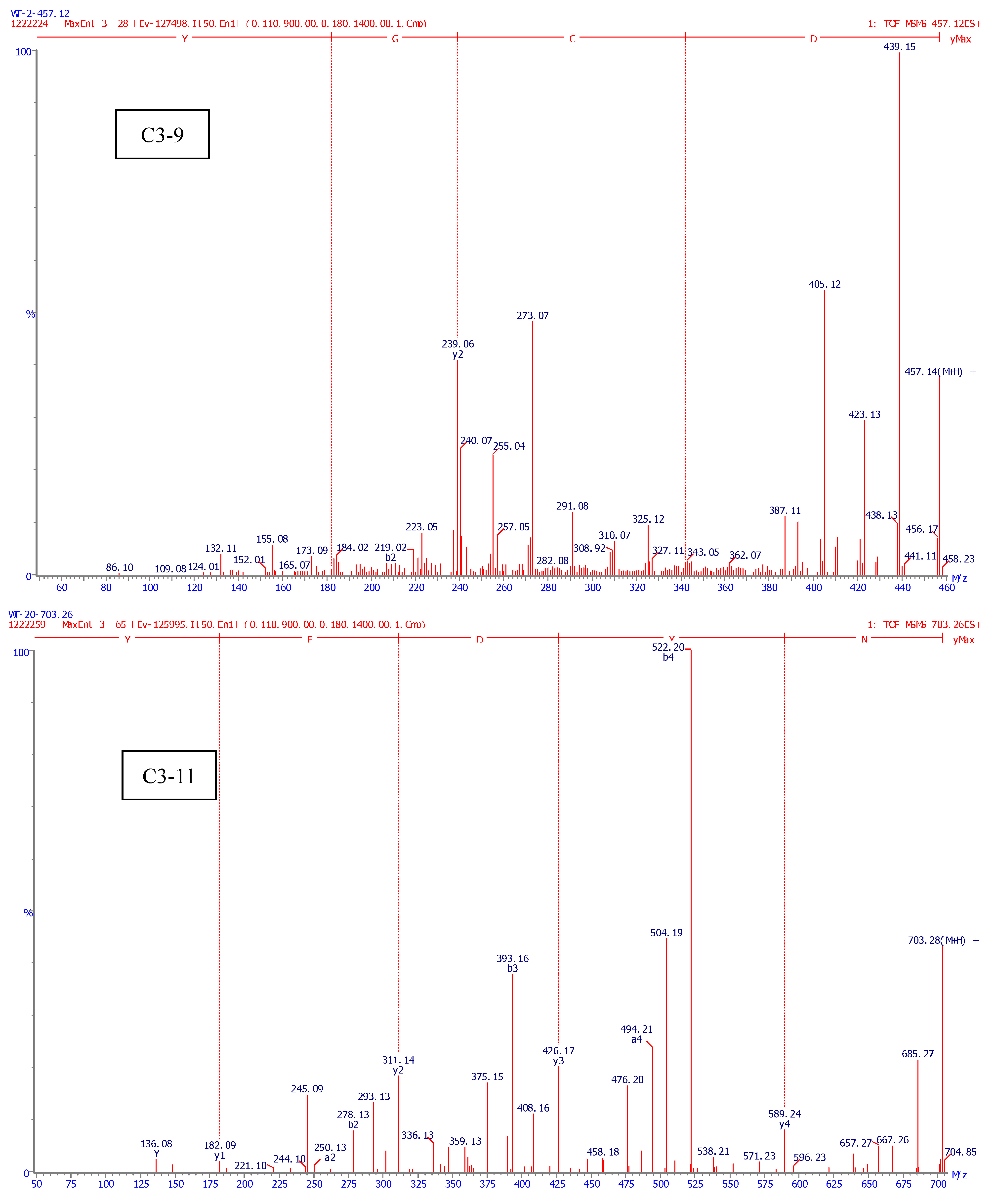
2.4. Identification of Amino Acid Sequence of Antioxidant Peptide
3. Expermental
3.1. Materials
3.2. Enzymatic Hydrolysis
| Enzyme | Activity (U/g) | Source | Buffer | pH | T (°C) | Time (h) | [E]/[S] (g/g) |
|---|---|---|---|---|---|---|---|
| Properase E | 6.5 × 104 | Bacillus | 0.05 M Na2HPO4-NaH2PO4 | 9.0 | 50 | 4 | 1:50 |
| Pepsin | 4.5 × 104 | Porcine stomach | 0.05 M Glycine-HCl | 2.0 | 37 | 6 | 1:50 |
| Trypsin | 9.5 × 104 | Bovine pancreas | 0.05 M Na2HPO4-NaH2PO4 | 7.5 | 45 | 3 | 1:100 |
| Flavorzyme | 5.5 × 104 | Aspergillus | 0.05 M Na2HPO4-NaH2PO4 | 7.0 | 45 | 4 | 1:100 |
| Neutrase | 2.0 × 105 | Bacillus | 0.05 M Na2HPO4-NaH2PO4 | 7.0 | 45 | 4 | 1:50 |
| GC106 | 5.0 × 104 | Aspergillus | 0.05 M Na2HPO4-NaH2PO4 | 4.5 | 45 | 6 | 1:33 |
| Papain | 8.5 × 104 | Papaya latex | 0.05 M Na2HPO4-NaH2PO4 | 6.0 | 37 | 3 | 1:100 |
3.3. Determination of the Degree of Hydrolysis
3.4. Antioxidant Activities Assay
3.4.1. 1,1-Diphenyl-2-picrylhydrazyl (DPPH) Radical Scavenging Activity Assay

3.4.2. Superoxide Anion (•O2) Scavenging Assay

3.4.3. Hydrogen Peroxide (H2O2) Scavenging Assay
3.4.4. Hydroxyl Radical (•OH) Scavenging Assay
3.5. Purification of Antioxidant Peptides
3.5.1. Ultrafiltration
3.5.2. Ion-Exchange Chromatography
3.5.3. Gel filtration Chromatography
3.5.4. High-Performance Liquid Chromatography (HPLC)
3.5.5. Amino Acid Sequence of the Purified Peptides
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
- Sample Availability: Samples of the tilapia frame protein hydrolysates and the peptide fractions are available from the authors.
References
- Hancock, J.T.; Desikan, R.; Neill, S.J. Role of reactive oxygen species in cell signaling pathways. Biochem. Soc. Trans. 2001, 29, 345–350. [Google Scholar] [CrossRef]
- Moskovitz, J.; Yim, K.A.; Choke, P.B. Free radicals and disease. Arch. Biochem. Biophys. 2002, 397, 354–359. [Google Scholar] [CrossRef]
- Qin, L.; Zhu, B.W.; Zhou, D.Y.; Wu, H.T.; Tan, H.; Yang, J.F.; Li, D.M.; Dong, X.P.; Murata, Y. Preparation and antioxidant activity of enzymatic hydrolysates from purple sea urchin (Strongylocentrotus nudus) gonad. LWT-Food Sci. Technol. 2011, 44, 1113–1118. [Google Scholar]
- Bougatef, A.; Hajji, M.; Balti, R.; Lassoued, I.; Triki-Ellouz, Y.; Nasri, M. Antioxidant and free radical-scavenging activities of smooth hound (Mustelus mustelus) muscle protein hydrolysates obtained by gastrointestinal proteases. Food Chem. 2009, 114, 1198–1205. [Google Scholar] [CrossRef]
- You, L.; Zhao, M.; Regenstein, J.M.; Ren, J. Purification and identification of antioxidant peptides from loach (Misgurnus anguillicaudatus) protein hydrolysate by consecutive chromatography and electrospray ionization-mass spectrometry. Food Res. Int. 2010, 43, 1167–1173. [Google Scholar] [CrossRef]
- Ali, B.; Naima, N.A.; Manni, L.; Ravallec, R.; Ahmed, B.; Didier, G. Purification and identification of novel antioxidant peptides from enzymatic hydrolysates of sardinelle (Sardinella aurita) by-products proteins. Food Chem. 2010, 118, 559–565. [Google Scholar] [CrossRef]
- Mendis, E.; Rajapakse, N.; Byun, H.G.; Kim, S.K. Investigation of jumbo squid (Dosidicus gigas) skin gelatin peptides for their in vitro antioxidant effects. Life Sci. 2005, 17, 2166–2178. [Google Scholar]
- Ren, J.; Zhao, M.; Shi, J.; Wang, J.; Jiang, Y.; Cui, C.; Kakuda, Y.; Xue, S.J. Purification and identification of antioxidant peptides from grass carp muscle hydrolysates by consecutive chromatography and electrospray ionization-mass spectrometry. Food Chem. 2008, 108, 727–736. [Google Scholar] [CrossRef]
- Kumar, N.S.S.; Nazeer, R.A.; Jaiganesh, R. Purification and biochemical characterization of antioxidant peptide from horse mackerel (Magalaspis cordyla) viscera protein. Peptides 2011, 32, 1496–1501. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, C.; Jiang, A. Antioxiant peptides isolated from sea cucumber Stichopus Japonicus. Eur. Food Res. Technol. 2012, 234, 441–447. [Google Scholar] [CrossRef]
- Jia, J.; Zhou, Y.; Lu, J.; Chen, A.; Li, Y.; Zheng, G. Enzymatic hydrolysis of Alaska pollack (Theragra chalcogramma) skin and antioxidant activity of the resulting hydrolysate. J. Sci. Food Agric. 2010, 90, 635–640. [Google Scholar]
- Cheung, I.W.Y.; Cheung, L.K.Y.; Tan, N.Y.; Li-Chan, E.C.Y. The role of molecular size in antioxidant activity of peptide fractions from Pacific hake (Merluccius productus) hydrolysates. Food Chem. 2012, 134, 1297–1306. [Google Scholar] [CrossRef]
- Kim, S.Y.; Je, J.Y.; Kim, S.K. Purification and characterization of antioxidant peptide from hoki (Johnius belengerii) frame protein by gastrointestinal digestion. J. Nutr. Biochem. 2007, 18, 31–38. [Google Scholar] [CrossRef]
- Je, J.Y.; Park, P.J.; Kim, S.K. Antioxidant activity of a peptide isolated from Alaska pollack (Theragra chalcogramma) frame protein hydrolysate. Food Res. Int. 2005, 38, 45–50. [Google Scholar] [CrossRef]
- Je, J.Y.; Qian, Z.J.; Byun, H.G.; Kim, S.K. Purification and characterization of an antioxidant peptide obtained from tuna backbone protein by enzymatic hydrolysis. Process Biochem. 2007, 42, 840–846. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Duan, X.; Zhuang, Y.L. Purification and characterization of novel antioxidant peptides from enzymatic hydrolysates of tilapia (Oreochromis niloticus) skin gelatin. Peptides 2012, 38, 13–20. [Google Scholar] [CrossRef]
- Jamilah, B.; Harvinder, K.G. Properties of gelatins from skins of fish black tilapia (Oreochromis mossambicus) and red tilapia (Oreochromis nilotica). Food Chem. 2002, 77, 81–84. [Google Scholar] [CrossRef]
- Ngo, D.H.; Qian, Z.J.; Ryu, B.M.; Park, J.W.; Kim, S.K. In vitro antioxidant activity of a peptide isolated from Nile tilapia (Oreochromis) scale gelatin in free radical-mediated oxidative systems. J. Funct. Foods 2010, 2, 107–117. [Google Scholar] [CrossRef]
- Dekkers, E.; Raghavan, S.; Kristinsson, H.G.; Marshall, M.R. Oxidative stability of mahi mahi red muscle dipped in tilapia protein hydrolysats. Food Chem. 2011, 124, 640–645. [Google Scholar] [CrossRef]
- Hou, H.; Fan, Y.; Li, B.F.; Xue, C.H.; Yu, G.L.; Zhang, Z.H.; Zhao, X. Purification and identification of immunomodulating peptides from enzymatic hydrolysates of Alaska Pollock frame. Food Chem. 2012, 134, 821–828. [Google Scholar] [CrossRef]
- Ghosh, R. Ultrafiltration: An overview. In Protein Bioseparation Using Ultrafiltration: Theory, Applications and New Developments; Ghosh, R., Ed.; Imperial College Press: London, UK, 2003; pp. 13–15. [Google Scholar]
- Moure, A.; Dominguez, H.; Parajo, J.C. Antioxidant properties of ultrafiltration recovered soy protein fractions from industrial effluents and their hydrolysates. Process Biochem. 2006, 41, 447–456. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, B.; Zhang, T.; Mu, W.; Liu, J. Antioxidant and free radical-scavenging activities of chickpea protein hydrolysate (CPH). Food Chem. 2008, 106, 444–450. [Google Scholar] [CrossRef]
- Sun, Q.; Luo, Y.; Shen, H.; Li, X.; Yao, L. Purification and characterization of a novel antioxidant peptides from porcine haemoglobin hydrolysate. Int. J. Food Sci. Technol. 2012, 47, 148–154. [Google Scholar] [CrossRef]
- Sampath Kumar, N.S.; Nazeer, R.A.; Jaiganesh, R. Purification and biochemical characterization of antioxidant peptide from horse mackerel (Magalaspis cordyla) viscera protein. Peptides 2011, 32, 149–156. [Google Scholar]
- Dong, S.Y.; Zeng, M.Y.; Wang, D.F.; Liu, Z.Y.; Zhao, Y.H.; Yang, H.C. Antioxidant and biochemical properties of protein hydrolysates prepared fromsilver carp (Hypophthalmichthys molitrix). Food Chem. 2007, 14, 1485–1493. [Google Scholar]
- Qian, Z.J.; Jung, W.K.; Byun, H.G.; Kim, S.K. Protective effect of an antioxidative peptide purified from gastrointestinal digests of oyster. Crassostrea gigas against free radical induced DNA damage. Bioresour. Technol. 2008, 99, 3365–3371. [Google Scholar] [CrossRef]
- Kawashima, K.; Itoh, H.; Miyoshi, M.; Chibata, I. Antioxidant properties of branched-chain amino acid derivatives. Chem. Pharm. Bull. 1979, 27, 1912–1916. [Google Scholar] [CrossRef]
- Yamaguchi, N.; Yokoo, Y.; Fujimaki, M. Studies on antioxidative activities of amino compounds on fats and oils. Part II. Antioxidative activities of dipeptides and their synergistic effects on tocopherol. Nippon Shokuhin Kogyo Gakkaishi 1975, 22, 425–430. [Google Scholar] [CrossRef]
- Park, P.J.; Jung, W.K.; Nam, K.S.; Shahidi, F.; Kim, S.K. Purification and characterization of antioxidative peptides from protein hydrolysate of lecithin-free egg yolk. J. Am. Oil Chem. Soc. 2001, 78, 651–656. [Google Scholar] [CrossRef]
- Zhang, J.H.; Zhang, H.; Wang, L.; Guo, X.N.; Wang, X.G.; Yao, H.Y. Isolation and identification of antioxidative peptides from rice endosperm protein enzymatic hydrolysate by consecutive chromatography and MALDI-TOF/TOF MS/MS. Food Chem. 2010, 119, 226–234. [Google Scholar] [CrossRef]
- Rajapakse, N.; Mendis, E.; Byun, H.G.; Kim, S.K. Purification and in vitro antioxidative effects of giant squid muscle peptides on free radical-mediated oxidative systems. J. Nutr. Biochem. 2005, 16, 562–569. [Google Scholar] [CrossRef]
- Li, X.D.; Niu, Z.X.; Zhang, B.L. Various methods available for the determination of hydrolyzed degree of whey protein. China Dairy Ind. 2006, 34, 59–62. [Google Scholar]
- Guo, Z.Y.; Liu, H.Y.; Chen, X.L.; Ji, X.; Li, P.C. Hydroxyl radicals scavenging activity of N-substituted chitosan and quaternized chitosan. Bioorg. Med. Chem. Lett. 2006, 16, 6348–6350. [Google Scholar]
- Huang, S.J.; Mau, J.L. Antioxidant properties of methanolic extracts from Agaricus blazei with various doses of c-irradiation. Food Sci. Technol. 2006, 39, 707–716. [Google Scholar]
- Zhao, X.; Xue, C.H.; Li, Z.J.; Cai, Y.P.; Liu, H.Y.; Qi, H.T. Antioxidant and hepato protective activities of low molecular weight sulfated polysaccharide from (Laminaria japonica). J. Appl. Phys. 2004, 16, 111–115. [Google Scholar]
- Zhuang, Y.L.; Sun, L.P.; Zhao, X.; Hou, H.; Li, B.F. Investigation of gelatin polypeptides of jellyfish (Rhopilema esculentum) for their antioxidant activity in vitro. Food Technol. Biotechnol. 2010, 48, 222–228. [Google Scholar]
- Lin, L.; Li, B.F. Radical scavenging properties of protein hydrolysates from Jumbo flying squid (Dosidicus eschrichitii Steenstrup) skin gelatin. J. Sci. Food Agric. 2006, 86, 2290–2295. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Fan, J.; He, J.; Zhuang, Y.; Sun, L. Purification and Identification of Antioxidant Peptides from Enzymatic Hydrolysates of Tilapia (Oreochromis niloticus) Frame Protein. Molecules 2012, 17, 12836-12850. https://doi.org/10.3390/molecules171112836
Fan J, He J, Zhuang Y, Sun L. Purification and Identification of Antioxidant Peptides from Enzymatic Hydrolysates of Tilapia (Oreochromis niloticus) Frame Protein. Molecules. 2012; 17(11):12836-12850. https://doi.org/10.3390/molecules171112836
Chicago/Turabian StyleFan, Jian, Jintang He, Yongliang Zhuang, and Liping Sun. 2012. "Purification and Identification of Antioxidant Peptides from Enzymatic Hydrolysates of Tilapia (Oreochromis niloticus) Frame Protein" Molecules 17, no. 11: 12836-12850. https://doi.org/10.3390/molecules171112836




