Stability Computations for Isomers of La@Cn (n = 72, 74, 76)
Abstract
:1. Introduction
2. Calculations

3. Results and Discussion
| Species b | ∆ Epot,rel | (kcal/mol) |
|---|---|---|
| B3LYP/3–21G~dz | B3LYP/6–31G*~dz | |
| IPR | 36.80 | 37.40 |
| C2v | 4.40 | 0.26 |
| C2 | 0 | 0 |





| Species b | ∆ Epot,rel | (kcal/mol) |
|---|---|---|
| B3LYP/3–21G ~dz | B3LYP/6–31G*~dz c | |
| 6727 | 15.86 | 19.46 |
| 1603 | 12.26 | 17.76 |
| 6247 | 10.00 | 13.22 |
| 6244 | 6.90 | 7.36 |
| T d IPR | 0 | 0 |
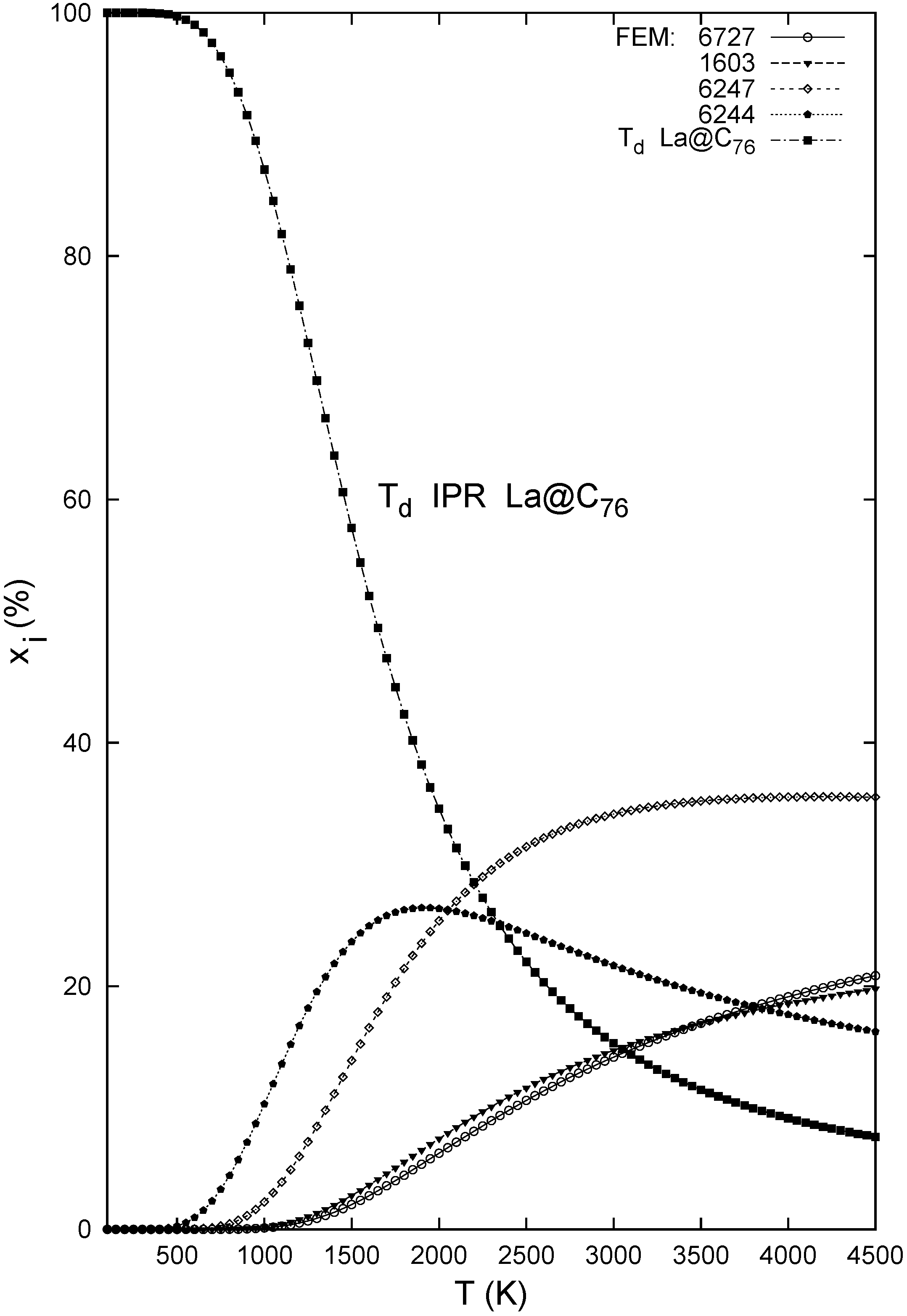
4. Conclusions
Acknowledgements
References
- Achiba, Y.; Kikuchi, K.; Aihara, Y.; Wakabayashi, T.; Miyake, Y.; Kainosho, M. Trends in Large Fullerenes: Are They Balls or Tubes. In The Chemical Physics of Fullerenes 10 (and 5) Years Later; Andreoni, W., Ed.; Kluwer Academic Publishers: Dordrecht, Netherlands, 1996; pp. 139–147. [Google Scholar]
- Diener, M.; Alford, J. Isolation and properties of small-bandgap fullerenes. Nature 1998, 393, 668–671. [Google Scholar] [CrossRef]
- Ichikawa, T.; Kodama, T.; Suzuki, S.; Fujii, R.; Nishikawa, H.; Ikemoto, I.; Kikuchi, K.; Achiba, Y. Isolation and characterization of a new isomer of Ca@C72. Chem. Lett. 2004, 33, 1008–1009. [Google Scholar] [CrossRef]
- Wakahara, T.; Nikawa, H.; Kikuchi, T.; Nakahodo, T.; Rahman, G.M.A.; Tsuchiya, T.; Maeda, Y.; Akasaka, T.; Yoza, K.; Horn, E.; et al. La@C72 having a non-IPR carbon cage. J. Am. Chem. Soc. 2006, 128, 14228–14229. [Google Scholar]
- Wan, T.; Zhang, H.; Nakane, T.; Xu, Z.; Inakuma, M.; Shinohara, H.; Kobayashi, K.; Nagase, S. Production, isolation, and electronic properties of missing fullerenes: Ca@C72 and Ca@C74. J. Am. Chem. Soc. 1998, 120, 6806–6807. [Google Scholar]
- Okazaki, T.; Lian, Y.; Gu, Z.; Suenaga, K.; Shinohara, H. Isolation and spectroscopic characterization of Sm-containing metallofullerenes. Chem. Phys. Lett. 2000, 320, 435–440. [Google Scholar] [CrossRef]
- Haufe, O.; Hecht, M.; Grupp, A.; Mehring, M.; Jansen, M. Isolation and spectroscopic characterization of new endohedral fullerenes in the sizegap of C74 to C76. Z. Anorg. Allgem. Chem. 2005, 631, 126–130. [Google Scholar] [CrossRef]
- Reich, A.; Panthofer, M.; Modrow, H.; Wedig, U.; Jansen, M. The structure of Ba@C74. J. Am. Chem. Soc. 2004, 126, 14428–14434. [Google Scholar] [CrossRef]
- Chai, Y.; Guo, T.; Jin, C.; Haufler, R.; Chibante, L.; Fure, J.; Wang, L.; Alford, J.; Smalley, R. Fullerenes with Metals Inside. J. Phys. Chem. 1991, 95, 7564–7568. [Google Scholar] [CrossRef]
- Nikawa, H.; Kikuchi, T.; Wakahara, T.; Nakahodo, T.; Tsuchiya, T.; Rahman, G.; Akasaka, T.; Maeda, Y.; Yoza, K.; Horn, E.; et al. Missing metallofullerene La@C74. J. Am. Chem. Soc. 2005, 127, 9684–9685. [Google Scholar]
- Matsuoka, H.; Ozawa, N.; Kodama, T.; Nishikawa, H.; Ikemoto, I.; Kikuchi, K.; Furukawa, K.; Sato, K.; Shiomi, D.; Takui, T.; et al. Multifrequency EPR study of metallofullerenes: Eu@C82 and Eu@C74. J. Phys. Chem. B 2004, 108, 13972–13976. [Google Scholar]
- Xu, J.; Lu, X.; Zhou, X.; He, X.; Shi, Z.; Gu, Z. Synthesis, isolation, and spectroscopic characterization of ytterbium-containing metallofullerenes. Chem. Mater. 2004, 16, 2959–2964. [Google Scholar] [CrossRef]
- Stevenson, S.; Dorn, H.; Burbank, P.; Harich, K.; Haynes, J.; Kiang, C.; Salem, J.; de Vries, M.; van Loosdrecht, P.; Johnson, R.; et al. Automated HPLC separation of endohedral metallofullerene Sc@C2n and Y@C2n Fractions. Anal. Chem. 1994, 66, 2675–2679. [Google Scholar] [CrossRef]
- Tagmatarchis, N.; Aslanis, E.; Prassides, K.; Shinohara, H. Mono-, di- and trierbium endohedral metallofullerenes: Production, separation, isolation, and spectroscopic study. Chem. Mater. 2001, 13, 2374–2379. [Google Scholar] [CrossRef]
- Moro, L.; Ruoff, R.; Becker, C.; Lorents, D.; Malhotra, R. Studies of metallofullerene primary soots by laser and thermal-desorption mass-spectrometry. J. Phys. Chem. 1993, 97, 6801–6805. [Google Scholar]
- Okubo, S.; Kato, T.; Inakuma, M.; Shinohara, H. Separation and characterization of ESR-active lanthanum endohedral fullerenes. New Diam. Front. Carbon Technol. 2001, 11, 285–294. [Google Scholar]
- Fowler, P.W.; Manolopoulos, D.E. An Atlas of Fullerenes; Clarendon Press: Oxford, UK, 1995. [Google Scholar]
- Kobayashi, K.; Nagase, S.; Yoshida, M.; Osawa, E. Endohedral metallofullerenes. Are the isolated pentagon rule and fullerene structures always satisfied? J. Am. Chem. Soc. 1997, 119, 12693–12694. [Google Scholar] [CrossRef]
- Nagase, S.; Kobayashi, K.; Akasaka, T. Unconventional cage structures of endohedral metallofullerenes. J. Mol. Struct. (Theochem) 1999, 461–462, 97–104. [Google Scholar] [CrossRef]
- Kobayashi, K.; Nagase, S. Structures and Electronic Properties of Endohedral Metallofullerenes: Theory and Experimen. In Endofullerenes – A New Family of Carbon Clusters; Akasaka, T., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002; pp. 99–119. [Google Scholar]
- Slanina, Z.; Kobayashi, K.; Nagase, S. Ca@C72 IPR and non-IPR structures: Computed temperature development of their relative concentrations. Chem. Phys. Lett. 2003, 372, 810–814. [Google Scholar] [CrossRef]
- Slanina, Z.; Kobayashi, K.; Nagase, S. Ca@C74 isomers: Relative concentrations at higher temperatures. Chem. Phys. 2004, 301, 153–157. [Google Scholar] [CrossRef]
- Tang, C.; Yuan, Y.; Deng, K.; Liu, Y.; Li, X.; Yang, J.; Wang, X. Geometric and electronic properties of endohedral Si@C74. J. Chem. Phys. 2006, 104307. [Google Scholar]
- Slanina, Z.; Uhlik, F.; Nagase, S. Computed structures of two known Yb@C74 isomers. J. Phys. Chem. A 2006, 110, 12860–12863. [Google Scholar]
- Slanina, Z.; Uhlik, F.; Lee, S.L.; Adamowicz, L.; Nagase, S. Computations on Three Isomers of La@C74. Int. J. Quantum Chem. 2008, 108, 2636–2640. [Google Scholar] [CrossRef]
- Slanina, Z.; Uhlik, F.; Adamowicz, L.; Nagase, S. Computational screening of metallofullerenes for nanoscience: Sr@C74. Mol. Simul. 2008, 34, 17–21. [Google Scholar] [CrossRef]
- Slanina, Z.; Uhlik, F. Temperature-dependence of the gibbs energy ordering of isomers of Cl2O2. J. Phys. Chem. 1991, 95, 5432–5434. [Google Scholar] [CrossRef]
- Slanina, Z.; Uhlik, F.; Zhao, X.; Osawa, E. Enthalpy-entropy interplay for C36 cages: B3LYP/6-31G(*) calculations. J. Chem. Phys. 2000, 113, 4933–4937. [Google Scholar] [CrossRef]
- Slanina, Z.; Kobayashi, K.; Nagase, S. Computed temperature development of the relative stabilities of La@C82 isomers. Chem. Phys. Lett. 2004, 388, 74–78. [Google Scholar] [CrossRef]
- Slanina, Z.; Nagase, S. Stability computations for Ba@C74 isomers. Chem. Phys. Lett. 2006, 422, 133–136. [Google Scholar] [CrossRef]
- Becke, A. Density-functional thermochemistry 3. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R. Development of the colle-salvetti correlation-energy formula into a functional of the electron-density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Hay, P.; Wadt, W. Ab initio effective core potentials for molecular calculations-potentials for Kto Au including the outermost core orbitals. J. Chem. Phys. 1985, 82, 299–310. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A., Jr.; Vreven, T.; Kudin, K.N.; Burant, J.C.; et al. Gaussian 03, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2004. [Google Scholar]
- Cao, X.; Dolg, M. Segmented contraction scheme for small-core lanthanide pseudopotential basis sets. J. Mol. Struct. (Theochem) 2002, 581, 139–147. [Google Scholar] [CrossRef]
- Slanina, Z. Equilibrium isomeric mixtures-potential-energy hypersurfaces as the origin of the overall thermodynamics and kinetics. Int. Rev. Phys. Chem. 1987, 6, 251–267. [Google Scholar] [CrossRef]
- Cross, R.; Saunders, M. Transmutation of fullerenes. J. Am. Chem. Soc. 2005, 127, 3044–3047. [Google Scholar] [CrossRef]
- Slanina, Z.; Adamowicz, L. On the relative stabilities of dodecahedron-shaped and bowl-shaped structures of C20. Thermochim. Acta 1992, 205, 299–306. [Google Scholar] [CrossRef]
- Slanina, Z.; Adamowicz, L.; Kobayashi, K.; Nagase, S. Gibbs energy-based treatment of metallofullerenes: Ca@C72, Ca@C74, Ca@C82, and La@C82. Mol. Simul. 2005, 31, 71–77. [Google Scholar] [CrossRef]
- Akasaka, T.; Nagase, S.; Kobayashi, K.; Walchli, M.; Yamamoto, K.; Funasaka, H.; Kako, M.; Hoshino, T.; Erata, T. C-13 and La-139 NMR studies of La2@C80: First evidence for circular motion of metal atoms in endohedral dimetallofullerenes. Angew. Chem. Intl. Ed. Engl. 1997, 36, 1643–1645. [Google Scholar] [CrossRef]
- Kobayashi, K.; Nagase, S.; Maeda, Y.; Wakahara, T.; Akasaka, T. La2@C80: Is the circular motion of two La atoms controllable by exohedral addition? Chem. Phys. Lett. 2003, 374, 562–566. [Google Scholar] [CrossRef]
- Slanina, Z.; Lee, S.L.; Uhlik, F.; Adamowicz, L.; Nagase, S. Computing relative stabilities of metallofullerenes by Gibbs energy treatments. Theor. Chem. Acc. 2007, 117, 315–322. [Google Scholar] [CrossRef]
- Gimzewski, J.K. Scanning Tunneling and Local Probe Studies of Fullerenes. In The Chemical Physics of Fullerenes 10 (and 5) Years Later; Andreoni, W., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1996; pp. 117–136. [Google Scholar]
- Harneit, W.; Waiblinger, M.; Meyer, C.; Lips, K.; Weidinger, A. Concept for Quantum Computing with N@C60. In Recent Advances in the Chemistry and Physics of Fullerenes and Related Materials, Volume 11, Fullerenes for the New Millennium; Kadish, K.M., Kamat, P.V., Guldi, D., Eds.; Electrochemical Society: Pennington, NJ, USA, 2001; pp. 358–361. [Google Scholar]
- Hiroshiba, N.; Tanigaki, K.; Kumashiro, R.; Ohashi, H.; Wakahara, T.; Akasaka, T. C60 field effect transistor with electrodes modified by La@C82. Chem. Phys. Lett. 2004, 400, 235–238. [Google Scholar] [CrossRef]
- Heine, T.; Vietze, K.; Seifert, G. C-13 NMR fingerprint characterizes long time-scale structure of Sc3N@C80 endohedral fullerene. Mag. Res. Chem. 2004, 42, S199–S201. [Google Scholar] [CrossRef]
- Slanina, Z.; Nagase, S. Sc3N@C80: Computations on the two-isomer equilibrium at high temperatures. ChemPhysChem 2005, 6, 2060–2063. [Google Scholar] [CrossRef]
- Liu, D.; Hagelberg, F. Impact of internal electron transfer on the structure of C74 encapsulating Sc and La metal atom impurities. Int. J. Quant. Chem. 2007, 107, 2253–2260. [Google Scholar] [CrossRef]
- Slanina, Z.; Ishimura, K.; Kobayashi, K.; Nagase, S. C72 isomers: The IPR-satisfying cage is disfavored by both energy and entropy. Chem. Phys. Lett. 2004, 384, 114–118. [Google Scholar] [CrossRef]
- Uhlik, F.; Slanina, Z.; Nagase, S. Mg@C74 isomers: Calculated relative concentrations and comparison with Ca@C74. Phys. Status Solidi A 2007, 204, 1905–1910. [Google Scholar] [CrossRef]
- Slanina, Z.; Uhlik, F.; Lee, S.L.; Adamowicz, L.; Nagase, S. Computed structures and relative stabilities of Be@C74. Int. J. Quant. Chem. 2007, 107, 2494–2498. [Google Scholar] [CrossRef]
- Slanina, Z.; Zhao, X.; Kurita, N.; Gotoh, H.; Uhlik, F.; Rudzinski, J.; Lee, K.; Adamowicz, L. Computing the relative gas-phase populations of C60 and C70: Beyond the traditional ∆Hf,298o scale. J. Mol. Graphics Mod. 2001, 19, 216–221. [Google Scholar] [CrossRef]
- Slanina, Z.; Lee, S.; Adamowicz, L.; Uhlik, F.; Nagase, S. Computed structure and energetics of La@C60. Int. J. Quant. Chem. 2005, 104, 272–277. [Google Scholar] [CrossRef]
- Rodriguez-Fortea, A.; Balch, A.L.; Poblet, J.M. Endohedral metallofullerenes: Aunique host-guest association. Chem. Soc. Rev. 2011, 40, 3551–3563. [Google Scholar] [CrossRef]
- Osuna, S.; Swart, M.; Sola, M. The reactivity of endohedral fullerenes. What can be learnt from computational studies? Phys. Chem. Chem. Phys. 2011, 13, 3585–3603. [Google Scholar]
- Yang, S.; Liu, F.; Chen, C.; Jiao, M.; Wei, T. Fullerenes encaging metal clusters-clusterfullerenes. Chem. Commun. 2011, 47, 11822–11839. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Slanina, Z.; Uhlík, F.; Lee, S.-L.; Adamowicz, L.; Akasaka, T.; Nagase, S. Stability Computations for Isomers of La@Cn (n = 72, 74, 76). Molecules 2012, 17, 13146-13156. https://doi.org/10.3390/molecules171113146
Slanina Z, Uhlík F, Lee S-L, Adamowicz L, Akasaka T, Nagase S. Stability Computations for Isomers of La@Cn (n = 72, 74, 76). Molecules. 2012; 17(11):13146-13156. https://doi.org/10.3390/molecules171113146
Chicago/Turabian StyleSlanina, Zdeněk, Filip Uhlík, Shyi-Long Lee, Ludwik Adamowicz, Takeshi Akasaka, and Shigeru Nagase. 2012. "Stability Computations for Isomers of La@Cn (n = 72, 74, 76)" Molecules 17, no. 11: 13146-13156. https://doi.org/10.3390/molecules171113146




