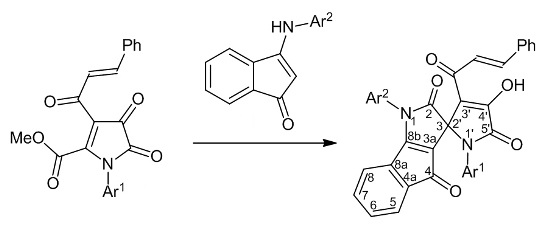
Spiroheterocyclization of Methyl 1-Aryl-3-cinnamoyl-4,5-dioxo-4,5-dihydro-1H-pyrrole-2-carboxylates by the Action of 3-(Arylamino)-1H-inden-1-ones
Abstract
:1. Introduction
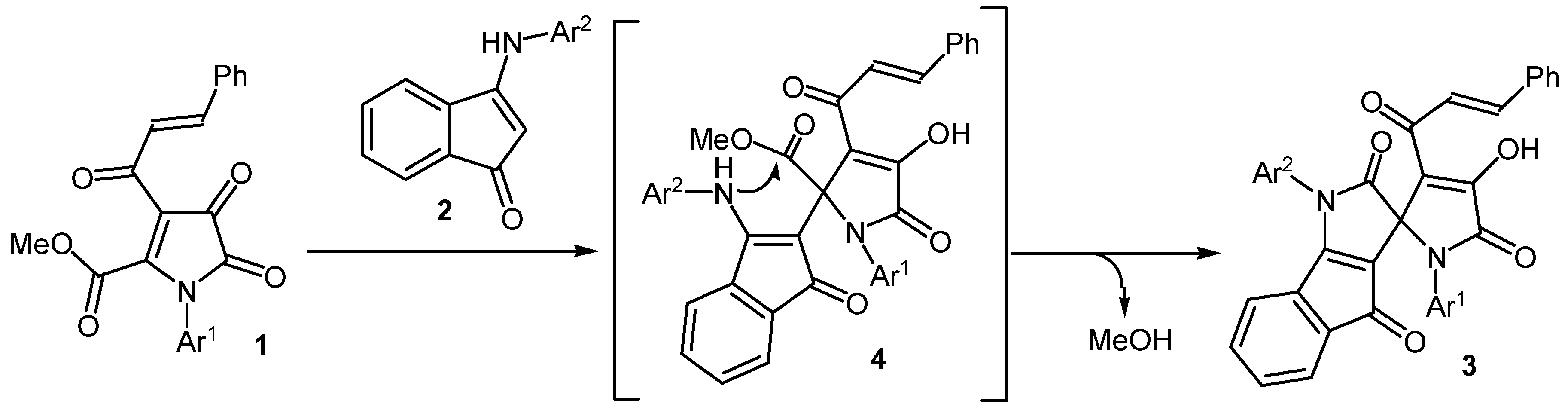
2. Results and Discussion
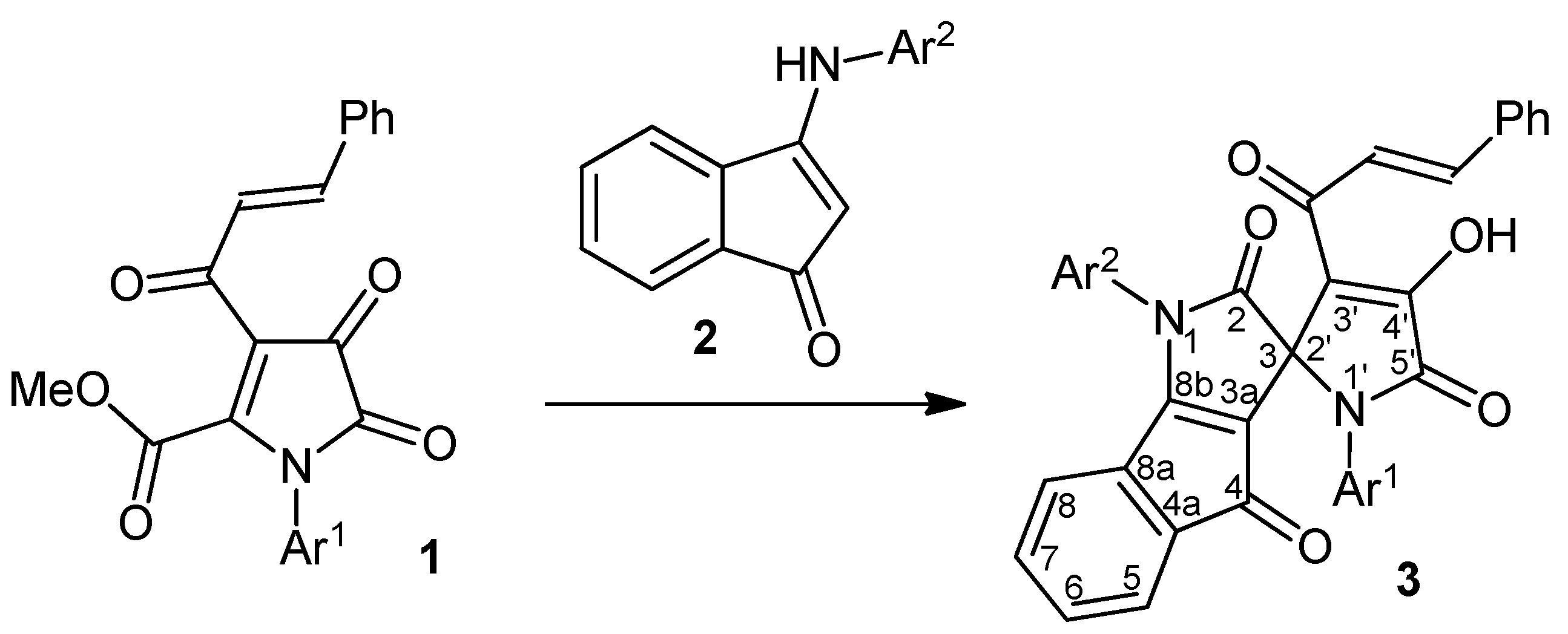
| Entry | Ar1 | Ar2 | Product 3 | Yield (%) |
|---|---|---|---|---|
| 1 | Ph | 4-BrC6H4 | 3a | 80 |
| 2 | 4-MeC6H4 | Ph | 3b | 82 |
| 3 | 4-MeC6H4 | 4-MeC6H4 | 3c | 79 |
| 4 | 4-MeC6H4 | 4-MeOC6H4 | 3d | 84 |
| 5 | 4-MeOC6H4 | Ph | 3e | 81 |
| 6 | 4-MeOC6H4 | 4-MeC6H4 | 3f | 80 |
| 7 | 4-MeOC6H4 | 4-MeOC6H4 | 3g | 79 |
| 8 | 4-MeOC6H4 | 4-BrC6H4 | 3h | 83 |
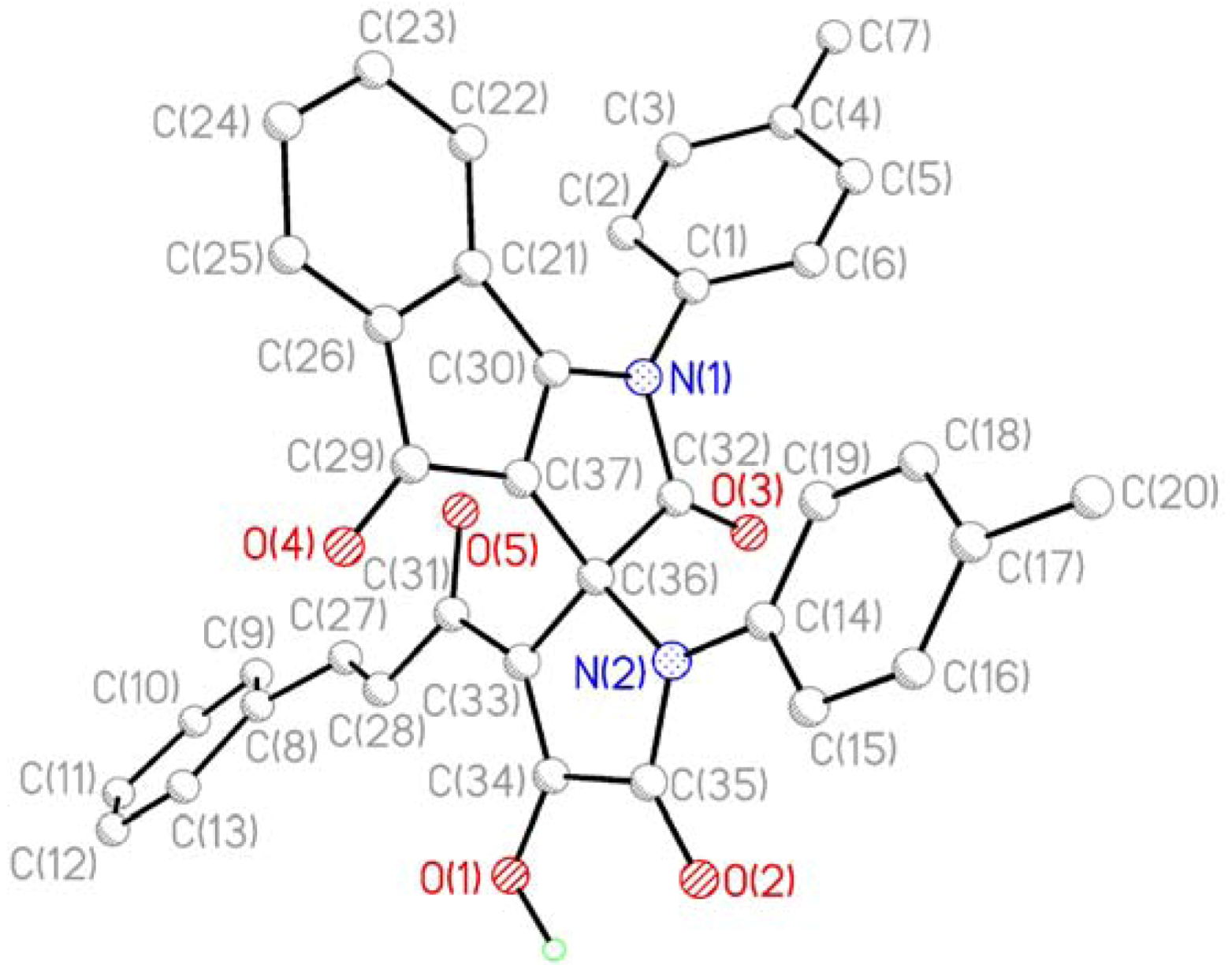
3. Experimental
3.1. General
3.2. General Procedure for Preparation of Spiro[indeno[1,2-b]pyrrole-3,2'-pyrroles] 3a–h
4. Conclusions
Acknowledgments
References
- Longeon, A.; Guyot, M.; Vacelet, J. Araplysillins-I and-II: Biologically active dibromotyrosine derivatives from the sponge Psammaplysilla arabica. Experentia 1990, 46, 548–550. [Google Scholar] [CrossRef]
- Kobayashi, J.; Tsuda, M.; Agemi, K.; Shigemiri, H.; Ishibashi, M.; Sasaki, T.; Mikami, Y. Purealidins B and C, new bromotyrosine alkaloids from the okinawan marine sponge psammaplysilla purea. Tetrahedron 1991, 47, 6617–6622. [Google Scholar] [CrossRef]
- James, D.M.; Kunze, H.B.; Faulkner, D.J. Two new brominated tyrosine derivatives from the sponge Druinella (=Psammaplysilla) purpurea. J. Nat. Prod. 1991, 54, 1137–1140. [Google Scholar] [CrossRef]
- The Alkaloids: Chemistry and Biology; Cordell, G.A. (Ed.) Academic: San Diego, CA, USA, 1998; Volume 5.
- Okita, T.; Isobe, M. Synthesis of the pentacyclic intermediate for dynemicin a and unusual formation of spiro-oxindole ring. Tetrahedron 1994, 50, 11143–11152. [Google Scholar] [CrossRef]
- Kornet, M.J.; Tnio, A.P. Oxindole-3-spiropyrrolidines and -piperidines. Synthesis and local anesthetic activity. J. Med. Chem. 1976, 19, 892–898. [Google Scholar] [CrossRef]
- Fisher, E.; Hirshberg, Y. Formation colored forms of spirans by low-temperature irradiation. J. Chem. Soc. 1952, 11, 4522–4530. [Google Scholar]
- Hinshberg, Y. Reversible Formation and Eradication of Colors by Irradiation at Low Temperatures. A Photochemical Memory Model. J. Am. Chem. Soc. 1956, 78, 2304–2312. [Google Scholar] [CrossRef]
- Berkovic, G.; Krongauz, V.; Weiss, V. Spiropyrans and Spirooxazines for Memories and Switches. Chem. Rev. 2000, 100, 1741–1754. [Google Scholar] [CrossRef]
- Bannikova, Y.N.; Sedegova, E.A.; Khalturina, V.V.; Maslivets, A.N. Five-membered 2,3-dioxo heterocycles: LV. Reaction of methyl 1-aryl-3-aroyl-4,5-dioxo-4,5-dihydro-1H-pyrrole-2-carboxylates with ethyl 3-arylaminobut-2-enoates. Russ. J. Org. Chem. 2007, 43, 1338–1341. [Google Scholar]
- Bannikova, Y.N.; Maslivets, A.N.; Aliev, Z.G. Five-membered 2,3-dioxo heterocycles: XLIX. Reaction of methyl 1-aryl-3-aroyl-4,5-dioxo-4,5-dihydro-1H-pyrrole-2-carboxylates with N-substituted 3-amino-5,5-dimethyl-2-cyclohexenones. Russ. J. Org. Chem. 2004, 40, 1791–1797. [Google Scholar]
- Silaichev, P.S.; Chudinova, M.A.; Slepukhin, P.A.; Maslivets, A. Five-membred 2,3-dioxoheterocycles: LXXXI. Reactions of 4,5-bis(methoxycarbonyl)-1H-pyrrole-2,3-diones with N-substituted 3-amino-5,5-dimethyl-2-cyclohex-2-en-1-ones. Crystal and molecular structure of methyl 4'-hydroxy-6,6-dimethyl-1,1'-bis(4-methylphenyl)-2,4,5'-trioxo-1,1',2,4,5,5',6,7-octahydrospiro [indole-3,2'-pyrrole]-3'-carboxylate. Russ. J. Org. Chem. 2011, 47, 1718–1722. [Google Scholar]
- Silaichev, P.S.; Filimonov, V.O.; Slepukhin, P.A.; Maslivets, A.N. Five-membered 2,3-dioxo heterocycles: LXXXV. Synthesis of methyl 1-aryl-4,5-dioxo-3-(1-oxo-3-phenylprop-2-en-1-yl)-4,5-dihydro-1H-pyrrole-2-carboxylates and their reaction with 3-amino-5,5-dimethylcyclohex-2-en-1-ones. Molecular and crystalline structure of 4'-hydroxy-1'-(4-methoxyphenyl)-6,6-dimethyl-3'-(1-oxo-3-phenylprop-2-en-1-yl)-1-phenyl-6,7-dihydrospiro[indole-3,2′-pyrrole]-2,4,5'(1H,1'H,5H)- trione. Russ. J. Org. Chem. 2012, 48, 561–565. [Google Scholar]
- Denislamova, E.S.; Bubnov, N.V.; Maslivets, A.N. Five-membered 2,3-dioxo heterocycles: LXXVI. Reaction of methyl 1-aryl-3-benzoyl-4,5-dioxo-4,5-dihydro-1H-pyrrole-2-carboxylates with 6-amino-1,3-dimethylpyrimidine-2,4(1H,3H)-dione. Russ. J. Org. Chem. 2011, 47, 933–936. [Google Scholar]
- Bannikova, Y.N.; Maslivets, A.N.; Rozhkova, Y.S.; Shklyaev, Y.V.; Aliev, Z.G. Spiro heterocyclization of 5-methoxycarbonyl-2,3-dihydro-2,3-pyrrolediones by reaction with 1-methyl-3,4-dihydroisoquinoline. Mendeleev Commun. 2005, 15, 158–159. [Google Scholar] [CrossRef]
- Bannikova, Y.N.; Rozhkova, Y.S.; Shklyaev, Y.V.; Maslivets, A.N. Five-membered 2,3-dioxoheterocycles: LVIII. Reaction of methyl 1-aryl-3-aroyl-4,5-dioxo-4,5-dihydro-1H-pyrrol-2-carboxylates with substituted 1-methyl-3,4-dihydroisoquinolines. New approach to the synthesis of steroid 13-azaanalogs. Russ. J. Org. Chem. 2008, 44, 697–700. [Google Scholar]
- Dmitriev, M.V.; Silaichev, P.S.; Aliev, Z.G.; Maslivets, A.N. Spiro-heterocyclization of 1H-pyrrole-2,3-dione at the treatment with 3-arylamino-1H-inden-1-ones. Russ. J. Org. Chem. 2011, 47, 304–305. [Google Scholar] [CrossRef]
- Spek, A.L. PLATON—A Multipurpose Crystallographic Tool, Utrecht University, Utrecht, The Netherlands. J. Appl. Cryst. 2003, 36, 7–13. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 1–3h are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Silaichev, P.S.; Filimonov, V.O.; Slepukhin, P.A.; Maslivets, А.N. Spiroheterocyclization of Methyl 1-Aryl-3-cinnamoyl-4,5-dioxo-4,5-dihydro-1H-pyrrole-2-carboxylates by the Action of 3-(Arylamino)-1H-inden-1-ones. Molecules 2012, 17, 13787-13794. https://doi.org/10.3390/molecules171213787
Silaichev PS, Filimonov VO, Slepukhin PA, Maslivets АN. Spiroheterocyclization of Methyl 1-Aryl-3-cinnamoyl-4,5-dioxo-4,5-dihydro-1H-pyrrole-2-carboxylates by the Action of 3-(Arylamino)-1H-inden-1-ones. Molecules. 2012; 17(12):13787-13794. https://doi.org/10.3390/molecules171213787
Chicago/Turabian StyleSilaichev, Pavel S., Valeriy O. Filimonov, Pavel A. Slepukhin, and Аndrey N. Maslivets. 2012. "Spiroheterocyclization of Methyl 1-Aryl-3-cinnamoyl-4,5-dioxo-4,5-dihydro-1H-pyrrole-2-carboxylates by the Action of 3-(Arylamino)-1H-inden-1-ones" Molecules 17, no. 12: 13787-13794. https://doi.org/10.3390/molecules171213787