Formation of Sulfonyl Aromatic Alcohols by Electrolysis of a Bisazo Reactive Dye
Abstract
:1. Introduction
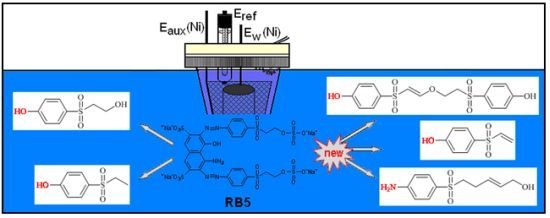
2. Results and Discussion
2.1. Identification of Products Formed by Electrolysis



| Compd. | Condensed formula | tR (min) | [M-H]exper. | [M-H]calc. | Δ m (ppm) | Reported by |
|---|---|---|---|---|---|---|
| 1 | C8H10O4S | 9.03 | 201.0227 | 201.0227 | 0.0 | [18] |
| 2 | C16H16O7S2 | 12.41 | 383.0260 | 383.0265 | −1.3 | new |
| 3 | C8H10O3S | 13.96 | 185.0278 | 185.0278 | 0.0 | [18] |
| 4 | C8H8O3S | 14.78 | 183.0121 | 183.0121 | 0.0 | new |
| 5 | C11H15NO3S | 15.70 | 240.0701 | 240.0700 | 0.4 | new |
| [M+H]exper. | [M+H]calc. | |||||
| MEBA | C9H13NO3S | 12.30 | 216.0691 | 216.0689 | 0.9 | [23] |
| Compd. | Proposed structure | [M-H]exper. | Product ions MS/MS |
|---|---|---|---|
| 1 | 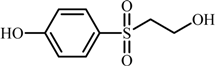 | 201.0227 | 92: [M-H-SO2C2H5O]−· |
| 108: [M-H-SOC2H5O]−· | |||
| 156: [M-H-C2H5OH]−· | |||
| 2 | 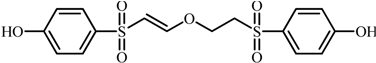 | 383.0260 | 353: [M-H-CH2O]−· |
| 262: [M-H-C3H5O3S]−· | |||
| 230: [M-H-C3H5O5S]−· | |||
| 199: [M-H-C8H8O3S]−· | |||
| 184: [M-H-C8H7O4S]−· | |||
| 169: [M-H-C9H10O4S]−· | |||
| 3 |  | 185.0278 | 92: [M-H-C2H5O2S]−· |
| 108: [M-H-C2H5OS]−· | |||
| 156: [M-H-C2H5]−· | |||
| 4 | 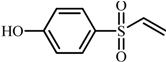 | 183.0121 | 92: [M-H-C2H5]−· |
| 108: [M-H-C2H3OS]−· | |||
| 156: [M-H-C2H5]−· | |||
| 5 |  | 240.0701 | 107: [M-H-C2H5NO2S]−· |
| 147: [M-H-CH3NO2S]−· | |||
| 163: [M-H-CHO2S]−· | |||
| 184: [M-H-C3H4O]−· | |||
| MEBA | 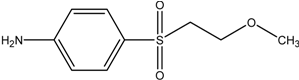 | [M+H]exper. 216.0691 |
2.2. Consideration of MEBA as One of the Unidentified Electrolysis Products

2.3. Hydrophobicity/Lipophilicity/Toxicity of the Decomposition Products

| Compound and IUPAC name | k | logkw,parab. (exper.) | logkw,lin. (exper.) | logkow (calc.) | m (Debye) | |
|---|---|---|---|---|---|---|
| 1 | 4-((2-hydroxyethyl)sulfonyl)phenol | 3.4 | 1.14 | 0.98 | 0.37 | 3.8 |
| MEBA | 4-(2-methoxyethylsulfonyl)benzenamine | 5.0 | 1.55 | 1.32 | 0.69 | 5.1 |
| 2 | 4-((2-(2-((4-hydroxyphenyl)sulfonyl)ethoxy)vinyl)sulfonyl)phenol | 5.1 | 1.30 | 1.17 | 0.76 | 5.6 |
| 3 | 4-(ethylsulfonyl)phenol | 5.8 | 1.93 | 1.67 | 1.18 | 5.5 |
| 4 | 4-(vinylsulfonyl)phenol | 6.2 | 2.34 | 1.92 | 1.03 | 6.7 |
| 5 | 5-((4-aminophenyl)sulfonyl)-2-penten-1-ol | 6.7 | 2.05 | 1.86 | 1.14 | 7.9 |
| Statistical parameters | k vs. logkw,linear | k vs. logkow | k vs. dipole moment |
|---|---|---|---|
| R2 | 0.9277 | 0.9448 | 0.9437 |
| SD | 0.1819 | 0.1273 | 0.5878 |
| RSS | 0.0993 | 0.0486 | 1.0364 |
| F | 18.52 | 24.96 | 24.43 |
| P | 0.0231 | 0.01543 | 0.01588 |
3. Experimental
3.1. General
3.1.1. Materials
3.1.2. Methods
3.1.3. General Analytical Instrumentation
3.1.4. Other Techniques and Instruments Used for the Thorough Characterization of MEBA
3.1.5. Calculations
3.2. Synthesis of 4-(2-Methoxyethylsulfonyl)benzenamine (MEBA)

3.3. Characterization of MEBA

4. Conclusions
Acknowledgments
- Sample Availability: Samples of the compound MEBA are available from the authors.
References
- Libra, J.A.; Borchert, M.; Vigelahn, L.; Storm, T. Two stage biological treatment of a diazo reactive textile dye and the fate of the dye metabolites. Chemosphere 2004, 56, 167–180. [Google Scholar] [CrossRef]
- Rehorek, A. Forschungsprojekt zur Verbesserung des Abbaus von Textilabwässern in einem anaerob/aerob Reaktor zur Vorbehandlung von Abwasserkonzentraten, Abschlussbericht Fachhochschule Köln; Project Report. University of Applied Sciences: Köln, Germany, 2006; 1–231. [Google Scholar]
- Rehorek, A.; Plum, A. Online LC-MS-MS process monitoring for optimization of biological treatment of wastewater containing azo dye concentrates. Anal. Bioanal. Chem. 2006, 384, 1123–1128. [Google Scholar] [CrossRef]
- Pham, T.L.H.; Weisshoff, H.; Mügge, C.; Krause, E.; Rotard, W.; Preiss, A.; Zaspel, I. Non-Target-Analytik in der Ökologie. Mitt. Umweltchem. Ökotox. 2010, 16, 2–8. [Google Scholar]
- Wang, K.-S.; Chen, H.-Y.; Huang, L.-Ch.; Su, Y.-Ch.; Chang, S.-H. Degradation of Reactive Black 5 using combined electrochemical degradation-solar-light/immobilized TiO2 film process and toxicity evaluation. Chemosphere 2008, 72, 299–305. [Google Scholar] [CrossRef]
- Riera-Torres, M.; Gutiérrez, M.-C. Colour removal of three reactive dyes by UV light exposure after electrochemical treatment. Chem. Eng. J. 2010, 156, 114–120. [Google Scholar] [CrossRef]
- Sakalis, A.; Ansorgová, D.; Holčapek, M.; Jandera, P.; Voulgaropoulos, A. Analysis of sulphonated azo dyes and their degradation products in aqueous solutions treated with a new electrochemical method. Int. J. Environ. Anal. Chem. 2004, 84, 875–888. [Google Scholar] [CrossRef]
- Vaněrková, D.; Sakalis, A.; Holčapek, M.; Jandera, P.; Voulgaropoulos, A. Analysis of electrochemical degradation products of sulphonated azo dyes using high-performance liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2006, 20, 2807–2815. [Google Scholar] [CrossRef]
- Raghu, S.; Basha, C.A. Dye destruction and simultaneous generation of sodium hydroxide using a divided electrochemical reactor. Ind. Eng. Chem. Res. 2008, 47, 5277–5283. [Google Scholar] [CrossRef]
- Soloman, P.A.; Basha, C.A.; Velan, M.; Ramamurthi, V.; Koteeswarwn, K.; Balasubramanian, N. Electrochemical degradation of Remazol Black B Dye effluent. Clean Soil Air Water 2009, 37, 889–900. [Google Scholar] [CrossRef]
- Martínez-Huitle, C.A.; Brillas, E. Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods: A general review. Appl. Catal. B Environ. 2009, 87, 105–145. [Google Scholar] [CrossRef]
- Panizza, M.; Cerisola, G. Direct and mediated anodic oxidation of organic pollutants. Chem. Rev. 2009, 109, 6541–6569. [Google Scholar] [CrossRef]
- Brillas, E.; Sirés, I.; Oturan, M.A. Electro-Fenton process and related electrochemical technologies based on Fenton´s reaction chemistry. Chem. Rev. 2009, 109, 6570–6631. [Google Scholar] [CrossRef]
- Mattusch, J.; Möller, D.; Elizalde-González, M.P.; Wennrich, R. Investigation of the degradation of arsenobetaine during its contact with natural zeolites and the identification of metabolites using HPLC coupled to ICP-MS and ESI-MS. Anal. Bioanal. Chem. 2008, 390, 1707–1715. [Google Scholar] [CrossRef]
- Elizalde-González, M.P.; Fuentes-Ramírez, L.E.; Guevara-Villa, M.R.G. Degradation of immobilized azo dyes by Klebsiella sp. UAP-b5 isolated from maize bioadsorbent. J. Hazard. Mater. 2009, 161, 769–774. [Google Scholar] [CrossRef]
- Cerón-Rivera, M.; Dávila-Jiménez, M.M.; Elizalde-González, M.P. Degradation of the textile dyes Basic yellow 28 and Reactive black 5 using diamond and metal alloys electrodes. Chemosphere 2004, 55, 1–10. [Google Scholar] [CrossRef]
- Méndez, M.A.; Tovar, G.R.; Dávila, M.M.; Ornelas, O.; Elizalde, M.P. Degradation of reactive black 5 and basic yellow 28 on metallic-polymer composites. Port. Electrochim. Acta 2008, 26, 89–100. [Google Scholar]
- Méndez-Martínez, A.J.; Dávila-Jiménez, M.M.; Ornelas-Dávila, O.; Elizalde-González, M.P.; Arroyo-Abad, U.; Sirés, I.; Brillas, E. Electrochemical reduction and oxidation pathways for Reactive Black 5 dye using nickel electrodes in divided and undivided cells. Electrochim. Acta 2012, 59, 140–149. [Google Scholar] [CrossRef]
- Schellenträger, M. Untersuchungen zur oxidativen Entfärbung ausgewählter Reaktivfarbstoffe: Analyse der Abbauprodukte mittels hochauflösender LC-MS.
- Bechtold, T.; Burtscher, E.; Turcanu, A. Cathodic decolourisation of textile waste water containing reactive dyes using a multi-cathode electrolyser. J. Chem. Technol. Biotechnol. 2001, 76, 303–311. [Google Scholar] [CrossRef]
- Rajkumar, D.; Kim, J.G. Oxidation various reactive dyes with in situ electro-generated active chlorine for textile dyeing industry wastewaters treatment. J. Hazard. Mater. 2006, 136, 203–212. [Google Scholar] [CrossRef]
- Sakalis, A.; Fytianos, K.; Nickel, U.; Voulgaropoulos, A. A comparative study of platinised titanium and niobe/synthetic diamond as anodes in the electrochemical treatment of textile wastewater. Chem. Eng. J. 2006, 119, 127–133. [Google Scholar] [CrossRef]
- Kitao, T.; Kuroki, N.; Konishi, K. On the synthesis of aryl vinyl sulfones and their reactive properties. Kogyo Kagaku Zasshi 1959, 62, 825–828. [Google Scholar] [CrossRef]
- Plum, A.; Rehorek, A. Strategies for continuous on-line high performance liquid chromatography coupled with diode array detection and electrospray tandem mass spectrometry for process monitoring of sulphonated azo dyes and their intermediates in anaerobic-aerobic bioreactors. J. Chromatogr. A 2005, 1084, 119–133. [Google Scholar] [CrossRef]
- Kudlich, M.; Hetheridge, M.J.; Knackmuss, H.-J.; Stolz, A. Autoxidation Reactions of Different Aromatic o-Aminohidroxynaphthalenes That Are Formed during the Anaerobic Reduction of Sulfonated Azo Dyes. Environ. Sci. Technol. 1999, 33, 896–901. [Google Scholar]
- Ishidate, M.; Noguchi, S.; Tamano, A. Studies on chemotherapeutics for acid-fast bacilli. VII. Further studies on p-aminophenyl- and p-aminomethylphenyl alkyl sulfones and related compounds. Pharm. Bull. 1953, 1, 287–289. [Google Scholar] [CrossRef]
- Janicka, M. Liquid chromatography in studying lipophylicity and bioactivity of pesticides. In Pesticides-Strategies for Pesticides Analysis; Stoytcheva, M., Ed.; In Tech: Rijeka, Croatia; New York, NY, USA; Shanghai, China, 2011; pp. 263–292. [Google Scholar]
- Hansch, C. Structure-activity relationships of chemical mutagens and carcinogens. Sci. Total Environ. 1991, 109, 17–29. [Google Scholar] [CrossRef]
- Cronin, M.T.D.; Zhao, Y.H.; Yu, R.L. pH-dependence and QSAR analysis of the toxicity of phenols and anilines to Daphnia magna. Environ. Toxicol. 2000, 15, 140–148. [Google Scholar] [CrossRef]
- Bączek, T.; Kaliszan, R. Predictive approaches to gradient retention based on analyte structural descriptors from calculation chemistry. J. Chromatogr. A 2003, 987, 29–37. [Google Scholar] [CrossRef]
- Ayouni, L.; Cazorla, G.; Chaillou, D.; Herbreteau, B.; Rudaz, S.; Lantéri, P.; Carrupt, P.-A. Fast Determination of Lipophilicity by HPLC. Chromatographia 2005, 62, 251–255. [Google Scholar] [CrossRef]
- Giaginis, C.; Theocharis, S.; Tsantili-Kakoulidou, A. Octanol/water partitioning simulation by reversed-phase high performance liquid chromatography for structurally diverse acidic drugs: Effect of n-octanol as mobile phase additive. J. Chromatogr. A 2007, 1166, 116–125. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Elizalde-González, M.P.; Arroyo-Abad, U.; García-Díaz, E.; Brillas, E.; Sirés, I.; Dávila-Jiménez, M.M. Formation of Sulfonyl Aromatic Alcohols by Electrolysis of a Bisazo Reactive Dye. Molecules 2012, 17, 14377-14392. https://doi.org/10.3390/molecules171214377
Elizalde-González MP, Arroyo-Abad U, García-Díaz E, Brillas E, Sirés I, Dávila-Jiménez MM. Formation of Sulfonyl Aromatic Alcohols by Electrolysis of a Bisazo Reactive Dye. Molecules. 2012; 17(12):14377-14392. https://doi.org/10.3390/molecules171214377
Chicago/Turabian StyleElizalde-González, María P., Uriel Arroyo-Abad, Esmeralda García-Díaz, Enric Brillas, Ignasi Sirés, and Martín M. Dávila-Jiménez. 2012. "Formation of Sulfonyl Aromatic Alcohols by Electrolysis of a Bisazo Reactive Dye" Molecules 17, no. 12: 14377-14392. https://doi.org/10.3390/molecules171214377