Production of Volatile Compounds in Reconstituted Milk Reduced-Fat Cheese and the Physicochemical Properties as Affected by Exopolysaccharide-Producing Strain
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Analysis
| Samples | Yield (%) | Protein (%) | Salt (%) | Moisture (%) | pH | Lactococci counts [LogCFU/g] |
|---|---|---|---|---|---|---|
| Direct-acidified cheese | ||||||
| Day 1 | 23.7 ± 0.3 a | 25.6 ± 0.4 a | 1.71 ± 0.08 a | 65.0 ± 0.8 ab | 6.32 ± 0.04 a | - |
| Day 21 | - | - | - | 62.5 ± 0.9 cd | 6.28 ± 0.05 a | - |
| Day 45 | - | - | - | 59.9 ± 1.3 e | 6.29 ± 0.06 a | - |
| Cheese with S. thermophilus TM11 | ||||||
| Day 1 | 24.8 ± 0.4 b | 23.9 ± 0.4 b | 1.73 ± 0.12 a | 66.5 ± 0.7 a | 5.13 ± 0.11 b | 7.7 ± 0.2 a |
| Day 21 | - | - | - | 66.3 ± 0.5 a | 5.08 ± 0.13 b | 7.8 ± 0.2 a |
| Day 45 | - | - | - | 65.9 ± 1.1 a | 5.01 ± 0.16 bc | 7.5 ± 0.3 a |
| Cheese with S. thermophilus SP1.1 | ||||||
| Day 1 | 23.5 ± 0.3 a | 25.3 ± 0.3 a | 1.74 ± 0.08a | 64.8 ± 0.7 abc | 4.98 ± 0.03 bc | 8.7 ± 0.3 b |
| Day 21 | - | - | - | 63.1 ± 0.6 bcd | 4.89 ± 0.03 bc | 8.7 ± 0.2 b |
| Day 45 | - | - | - | 62.1 ± 0.7 de | 4.80 ± 0.06 c | 8.6 ± 0.3 b |
2.2. Volatile Compounds in CRMP
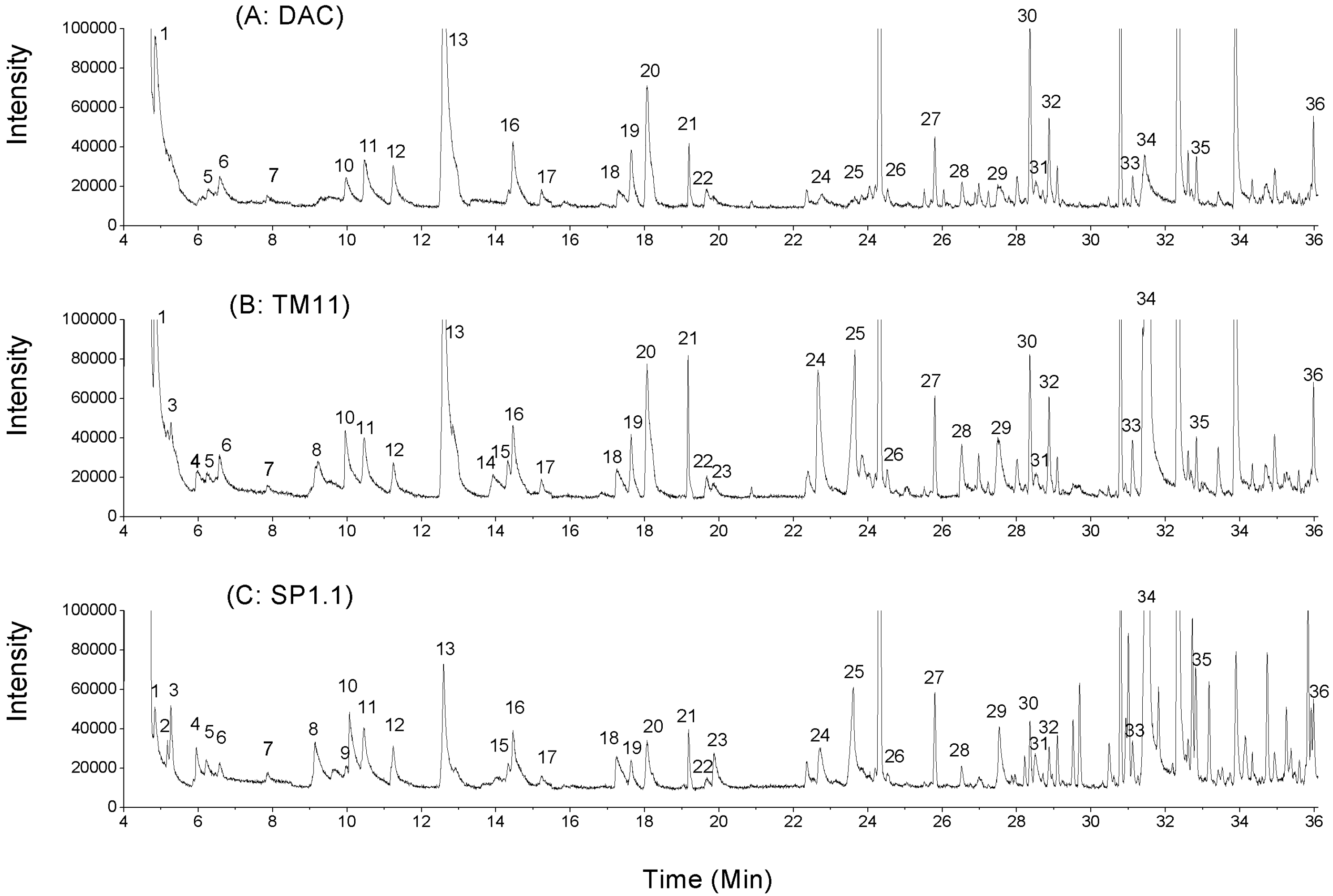
| Peak number * | Retention time, min | Volatiles | AR | ||
|---|---|---|---|---|---|
| DAC | TM11 | SP1.1 | |||
| 1 | 4.84 | Acetone | 5.80 ± 1.46 a | 7.24 ± 3.57 a | 2.30 ± 0.94 b |
| 2 | 5.17 | Thiourea | 0.41 ± 0.35 a | 0.39 ± 0.47 a | 0.59 ± 0.27 a |
| 3 | 5.26 | Carbon disulfide | 0.79 ± 0.83 a | 1.29 ± 0.77 a | 1.67 ± 0.85 a |
| 4 | 5.95 | 2,3-Butanedione | - | 1.02 ± 0.41 a | 0.84 ± 0.29 a |
| 5 | 6.22 | 2-Butanone | 0.56 ± 0.68 a | 0.42 ± 0.57 a | 0.62 ± 0.81 a |
| 6 | 6.58 | Ethyl acetate | 1.11 ± 0.89 a | 1.66 ± 1.41 a | 0.54 ± 0.33 a |
| 7 | 7.85 | Benzene | 0.03 ± 0.02 a | 0.04 ± 0.03 a | 0.08 ± 0.04 a |
| 8 | 9.15 | 2,3-Pentanedione | - | 3.58 ± 1.83 a | 2.34 ± 1.06 a |
| 9 | 9.99 | 3-Hydroxy-2-butanone | - | - | 0.36 ± 0.22 |
| 10 | 10.07 | Methyl 2-methyl-2-propenoate | 1.17 ± 1.22 a | 2.03 ± 1.14 a | 2.50 ± 2.19 a |
| 11 | 10.45 | Methyl butyrate | 2.78 ± 0.99 a | 3.12 ± 0.80 a | 2.59 ± 1.25 a |
| 12 | 11.24 | 4-Methyl-2-pentanone | IS | IS | IS |
| 13 | 12.79 | Toluene | 9.92 ± 3.57 a | 16.76 ± 4.80 b | 7.71 ± 4.48 a |
| 14 | 13.98 | Butyric acid | - | 1.52 ± 1.46 a | 0.05 ± 0.04 b |
| 15 | 14.33 | Hexanal | 0.15 ± 0.24 a | 0.93 ± 0.36 b | 0.34 ± 0.28 a |
| 16 | 14.46 | Ethyl butyrate | 4.97 ± 3.43 a | 6.12 ± 3.49 a | 3.69 ± 1.59 a |
| 17 | 15.22 | Butyl acetate | 0.97 ± 0.51 a | 0.70 ± 0.26 a | 0.58 ± 0.21 a |
| 18 | 17.25 | 2-Hexenal | 0.59 ± 0.22 a | 1.70 ± 0.64 b | 0.51 ± 0.45 a |
| 19 | 17.63 | Ethylbenzene | 1.62 ± 0.84 a | 1.85 ± 0.51 a | 0.42 ± 0.36 b |
| 20 | 18.07 | Xylene | 4.64 ± 2.95 a | 5.42 ± 2.44 a | 1.22 ± 1.93 b |
| 21 | 19.16 | 2-Heptanone | 1.82 ± 1.36 a | 2.45 ± 0.86 a | 0.57 ± 0.34 b |
| 22 | 19.68 | Heptanal | 0.77 ± 0.48 a | 0.54 ± 0.23 a | 0.14 ± 0.17 b |
| 23 | 19.94 | Methoxy-phenyl-oxime | - | 0.26 ± 0.14 a | 0.92 ± 0.56 b |
| 24 | 22.71 | Benzaldehyde | 1.13 ± 1.93 a | 6.00 ± 2.09 b | 1.22 ± 0.54 a |
| 25 | 23.61 | Hexanoic acid | 0.31 ± 0.15 a | 8.35 ± 3.55 b | 3.10 ± 1.43 c |
| 26 | 24.54 | Octanal | 0.57 ± 0.20 a | 0.92 ± 0.39 b | 0.20 ± 0.14 a |
| 27 | 25.80 | Limonene | 1.22 ± 0.53 a | 1.37 ± 0.38 a | 1.26 ± 0.76 a |
| 28 | 26.52 | Indene | 0.91 ± 0.36 a | 1.21 ± 0.42 a | 0.41 ± 0.32 b |
| 29 | 27.53 | Acetophenone | 0.43 ± 0.29 a | 2.70 ± 1.20 b | 1.48 ± 0.68 ab |
| 30 | 28.36 | 2-Nonanone | 2.46 ± 1.38 a | 2.91 ± 1.25 a | 0.88 ± 0.62 b |
| 31 | 28.50 | 3,5-Octadien-2-one | 0.51 ± 0.43 a | 0.65 ± 0.31 a | 0.41 ± 0.27 a |
| 32 | 28.87 | Nonanal | 1.94 ± 0.84 a | 1.85 ± 0.76 a | 0.57 ± 0.13 b |
| 33 | 31.12 | 2-Nonenal | 1.15 ± 0.80 a | 1.83 ± 1.09 a | 0.79 ± 0.85 a |
| 34 | 31.47 | Octanoic acid | 4.40 ± 2.29 a | 17.97 ± 5.92 b | 14.23 ± 4.88 b |
| 35 | 32.81 | Decanal | 1.40 ± 1.37 a | 1.72 ± 0.79 a | 1.84 ± 1.04 a |
| 36 | 35.98 | 2-Undecanone | 1.34 ± 0.78 a | 1.80 ± 0.74 a | 1.09 ± 0.51 a |
2.2.1. Ketones
2.2.2. Aldehydes
2.2.3. Acids, Alcohols and Esters
2.2.4. Sulfur-Containing Compounds
2.2.5. Other Volatiles
2.3. Texture of CRMP
2.3.1. The Effect of Storage Time

2.3.2. The Effect of Starter Cultures
2.4. Microstructure of CRMP

3. Experimental
3.1. Cheese Manufacture
3.2. Physicochemical and Microbiological Analyses
3.3. Volatile Compounds
3.4. Textural Profile Analysis (TPA) of Cheese
3.5. Microstructure
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Conflict of Interest
- Sample Availability: Not Available.
References
- Zhao, Z.; Chang, Z.Y.; Shen, S.H.; Wei, W.; Li, X. Product strategy for Chinese cheese development. China Food Ind. 2007, 2, 28–30. [Google Scholar]
- Gee, P.; Townson, P. Cheese if you please: Getting the Chinese market to consume cheese is a challenge in a lactose-intolerant population. Dairy Ind. Int. 2007, 72, 18–21. [Google Scholar]
- Zhang, X.Y.; Guo, H.Y.; Zhao, L.; Sun, W.F.; Zeng, S.S.; Lu, X.M.; Cao, X.; Ren, F.Z. Sensory profile and Beijing youth preference of seven cheese varieties. Food Qual. Preference 2011, 22, 101–109. [Google Scholar] [CrossRef]
- Yuceer, Y.K.; Tuncel, B.; Guneser, O.; Engin, B.; Isleten, M.; Yasar, K.; Mendes, M. Characterization of aroma-active compounds, sensory properties, and proteolysis in Ezine cheese. J. Dairy Sci. 2009, 92, 4146–4157. [Google Scholar]
- Mistry, V.V. Low fat cheese technology. Int. Dairy J. 2001, 11, 413–422. [Google Scholar] [CrossRef]
- Manolaki, P.; Katsiari, M.C.; Alichanidis, E. Effect of a commercial adjunct culture on organic acid contents of low-fat Feta-type cheese. Food Chem. 2006, 98, 658–663. [Google Scholar] [CrossRef]
- Zisu, B.; Shah, N.P. Texture characteristics and pizza bake properties of low-fat Mozzarella cheese as influenced by pre-acidification with citric acid and use of encapsulated and ropy exopolysaccharide producing cultures. Int. Dairy J. 2007, 17, 985–997. [Google Scholar] [CrossRef]
- Katsiari, M.C.; Voutsinas, L.P.; Kondyli, E. Improvement of sensory quality of lowfat Kefalograviera-type cheese by using commercial special starter cultures. J. Dairy Sci. 2002, 85, 2759–2767. [Google Scholar] [CrossRef]
- Perry, D.B.; McMahon, D.J.; Oberg, C.J. Effect of exopolysaccharide producing cultures on moisture retention in low-fat Mozzarella cheese. J. Dairy Sci. 1997, 80, 799–805. [Google Scholar] [CrossRef]
- Low, D.; Ahlgren, J.A.; Horne, D.; McMahon, D.J.; Oberg, C.J.; Broadbent, J.R. Role of Streptococcus thermophilus MR-1C capsular exopolysaccharide in cheese moisture retention. Appl. Environ. Microbiol. 1998, 64, 2147–2151. [Google Scholar]
- Broadbent, J.R.; McMahon, D.J.; Oberg, C.J.; Welker, D.L. Use of exopolysaccharide-producing cultures to improve the functionality of low fat cheese. Int. Dairy J. 2001, 11, 433–439. [Google Scholar] [CrossRef]
- Petersen, B.L.; Dave, R.I.; McMahon, D.J.; Oberg, C.J.; Broadbent, J.R. Influence of capsular and ropy exopolysaccharide-producing Streptococcus thermophilus on Mozzarella cheese and cheese whey. J. Dairy Sci. 2000, 83, 1952–1956. [Google Scholar] [CrossRef]
- Dabour, N.; Kheadr, E.; Benhamou, N.; Fliss, I.; LaPointe, G. Improvement of texture and structure of reduced-fat Cheddar cheese by exopolysaccharide-producing Lactococci. J. Dairy Sci. 2006, 89, 95–110. [Google Scholar] [CrossRef]
- Awad, S.; Hassan, A.N.; Muthukumarappan, K. Application of exopolysaccharide-producing cultures in reduced-fat Cheddar cheese: Texture and melting properties. J. Dairy Sci. 2005, 88, 4204–4213. [Google Scholar] [CrossRef]
- Mistry, V.V.; Anderson, D.L. Composition and microstructure of commercial full-fat and low-fat cheeses. Food Struct. 1993, 12, 259–266. [Google Scholar]
- Tamime, A.Y.; Kalab, M.; Davies, G.; Younis, M.F. Microstructure and firmness of processed cheese manufactured from Cheddar cheese and skim milk powder cheese base. Food Struct. 1990, 9, 23–37. [Google Scholar]
- Leclercq-Perlat, M.N.; Oumer, A.; Bergere, J.L.; Spinnler, H.E. Behavior of Brevibacterium linens and Debaryomyces hansenii as ripening flora in controlled production of smear soft cheese from reconstituted milk: Growth and substrate consumption. J. Dairy Sci. 2000, 83, 1665–1673. [Google Scholar] [CrossRef]
- Leclercq-Perlat, M.N.; Oumer, A.; Buono, F.; Bergere, J.L. Behavior of Brevibacterium linens and Debaryomyces hansenii as ripening flora in controlled production of soft smear cheese from reconstituted milk: Protein degradation. J. Dairy Sci. 2000, 83, 1674–1683. [Google Scholar] [CrossRef]
- Wacher-Rodarte, C.; Galvan, M.V.; Farres, A.; Gallardo, F.; Marshall, V.M.E.; Garcia-Garibay, M. Yogurt production from reconstituted skim milk powders using different polymer and non-polymer forming starter cultures. J. Dairy Res. 1993, 60, 247–254. [Google Scholar] [CrossRef]
- Amatayakul, T.; Sherkat, F.; Shah, N.P. Syneresis in set yogurt as affected by EPS starter cultures and levels of solids. Int. J. Dairy Technol. 2006, 59, 216–221. [Google Scholar] [CrossRef]
- Jimenez-Guzman, J.; Flores-Najera, A.; Cruz-Guerrero, A.E.; Garcia-Garibay, M. Use of an exopolysaccharide-producing strain of Streptococcus thermophilus in the manufacture of Mexican Panela cheese. LWT-Food Sci. Technol. 2009, 42, 1508–1512. [Google Scholar] [CrossRef]
- Molimard, P.; Spinnler, H.E. Review: Compounds involved in the flavour of surface mold-ripened cheeses: Origins and properties. J. Dairy Sci. 1996, 79, 169–184. [Google Scholar] [CrossRef]
- Dartey, C.; Kinsella, K.; Kinsella, J.E. Metabolism of carbon-14-uniformly-labeled lauric acid to methyl ketones by the spores of Penicillium roqueforti. J. Agric. Food Chem. 1973, 21, 933–936. [Google Scholar] [CrossRef]
- Starrenburg, M.J.C.; Hugenholtz, J. Citrate fermentation by Lactococcus and Leuconostoc spp. Appl. Environ. Microbiol. 1991, 57, 3535–3540. [Google Scholar]
- Jordan, K.N.; Cogan, T.M. Production of acetolactate by Streptococcus diacetylactis and Leuconostoc spp. J. Dairy Res. 1988, 55, 227–238. [Google Scholar] [CrossRef]
- Bintsis, T.; Robinson, R.K. A study of the effects of adjunct cultures on the aroma compounds of Feta-type cheese. Food Chem. 2004, 88, 435–441. [Google Scholar] [CrossRef]
- Massouras, T.; Papa, E.C.; Mallatou, H. Headspace analysis of volatile flavour compounds of Teleme cheese made from sheep and goat milk. Int. J. Dairy Techol. 2006, 59, 250–256. [Google Scholar] [CrossRef]
- Bosset, J.O.; Gauch, G. Comparison of the volatile flavour compounds of six European “AOC” cheeses using a new dynamic headspace GC-MS method. Int. Dairy J. 1993, 3, 423–460. [Google Scholar] [CrossRef]
- Engels, W.J.M.; Dekker, R.; de Jong, C.; Neeter, R.; Visser, S. A comparative study of volatile compounds in the water-soluble fraction of various types of ripened cheese. Int. Dairy J. 1997, 7, 255–263. [Google Scholar] [CrossRef]
- Centeno, J.A.; Tomillo, F.J.; Fernandez-Garcia, E.; Gaya, P.; Nunez, M. Effect of wild strains of Lactococcus lactis on the volatile profile and the sensory characteristics of ewes’ raw milk cheese. J. Dairy Sci. 2002, 85, 3164–3172. [Google Scholar] [CrossRef]
- Carpino, S.; Malli, S.; La Terra, S.; Melilli, C.; Licitra, G.; Acree, T.E.; Barbano, D.M.; van Soest, P.J. Composition and aroma compounds of Ragusano cheese: Native pasture and total mixed rations. J. Dairy Sci. 2004, 87, 816–830. [Google Scholar] [CrossRef]
- Fox, P.F.; Wallace, J.M. Formation of flavorcompounds in cheese. Adv. Appl. Microbiol. 1997, 45, 17–85. [Google Scholar] [CrossRef]
- Urbach, G. Contribution of lactic acid bacteria to flavour compounds formation in dairy products. Int. Dairy J. 1995, 5, 877–903. [Google Scholar] [CrossRef]
- Morgan, M.E. Microbial flavor defects in dairy products and methods for their simulation. I. Malty flavor. J. Dairy Sci. 1970, 53, 270–272. [Google Scholar] [CrossRef]
- Urbach, G. Relations between cheese flavour and chemical composition. Int. Dairy J. 1993, 3, 389–422. [Google Scholar] [CrossRef]
- Sheldon, R.M.; Lindsay, R.C.; Libbey, L.M.; Morgan, M.E. Chemical nature of malty flavor and aroma produced by Streptococcus lactis var. maltigenes. Appl. Environ. Microbiol. 1971, 22, 263–266. [Google Scholar]
- Arora, G.; Cormier, F.; Lee, B. Analysis of odor-active volatiles in Cheddar cheese headspace by multidimensional GC/MS/Sniffing. J. Agric. Food Chem. 1995, 43, 748–752. [Google Scholar] [CrossRef]
- Fernandez-Garcia, E. Use of headspace sampling in the quantitative analysis of Artisanal Spanish cheese aroma. J. Agric. Food Chem. 1996, 44, 1833–1839. [Google Scholar] [CrossRef]
- Burbanka, H.; Qian, M.C. Development of volatile sulfur compounds in heat-shocked and pasteurized milk cheese. Int. Dairy J. 2008, 18, 811–818. [Google Scholar] [CrossRef]
- Hall, G.; Andersson, J. Flavor changes in whole milk powder during storage. J. Food Qual. 1985, 7, 237–253. [Google Scholar] [CrossRef]
- Vazquez-Landaverde, P.A.; Torres, J.A.; Qian, M.C. Quantification of trace volatile sulfur compounds in milk by solid-phase microextraction and gas chromatography–pulsed flame. J. Dairy Sci. 2006, 89, 2919–2927. [Google Scholar] [CrossRef]
- Cornu, A.; Kondjoyan, N.; Martin, B.; Verdier-Metz, I.; Pradel, P.; Berdague, J.L.; Coulon, J.B. Terpene profiles in Cantal and Saint-Nectaire-type cheese made from raw or pasteurised milk. J. Sci. Food Agric. 2005, 85, 2040–2046. [Google Scholar]
- Solano-Lopez, C.E.; Ji, T.; Alvarez, V.B. Volatile compounds and chemical changes in ultrapasteurized milk packaged in polyethylene terephthalate containers. J. Food Sci. 2005, 70, 407–412. [Google Scholar]
- Pappa, E.C.; Kandarakis, I.; Mallatou, H. Effect of different types of milks and cultures on the rheological characteristics of Teleme cheese. J. Food Eng. 2007, 79, 143–149. [Google Scholar] [CrossRef]
- Fox, P.F.; O’Connor, T.P.; McSweeney, P.L.H. Cheese: Physical, biochemical and nutritional aspects. Adv. Food Nutr. Res. 1996, 39, 163–328. [Google Scholar] [CrossRef]
- Liu, H.; Xu, X.M.; Guo, S.D. Comparison of full-fat and low-fat cheese analogues with or without pectin gel through microstructure, texture, rheology, thermal and sensory analysis. Int. J. Food Sci. Tech. 2008, 43, 1581–1592. [Google Scholar] [CrossRef]
- Lopez, C.; Camiera, B.; Gassia, J.Y. Development of the milk fat microstructure during the manufacture and ripening of Emmental cheese observed by confocal laser scanning microscopy. Int. Dairy J. 2007, 17, 235–247. [Google Scholar] [CrossRef]
- Hassan, A.N.; Frank, J.F.; Corredig, M. Microstructure of feta cheese made using different cultures as determined by confocal scanning laser microscopy. J. Food Sci. 2002, 67, 2750–2753. [Google Scholar]
- Association of Official Analytical Chemists International, Official Methods of Analysis of AOAC, 17th ed; AOAC Int.: Gaithersburg, MD, USA, 2000.
- Guinee, T.P.; Auty, M.A.E.; Fenelon, M.A. The effect of fat content on the rheology, microstructure and heat-induced functional characteristics of Cheddar cheese. Int. Dairy J. 2000, 10, 277–288. [Google Scholar] [CrossRef]
- Serrano, J.; Velazquez, G.; Lopetcharat, K.; Ramirez, J.A.; Torres, J.A. Effect of moderate pressure treatments on microstructure, texture, and sensory properties of stirred-curd cheddar shreds. J. Dairy Sci. 2004, 87, 3172–3182. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, W.; Zhang, L.; Li, Y. Production of Volatile Compounds in Reconstituted Milk Reduced-Fat Cheese and the Physicochemical Properties as Affected by Exopolysaccharide-Producing Strain. Molecules 2012, 17, 14393-14408. https://doi.org/10.3390/molecules171214393
Wang W, Zhang L, Li Y. Production of Volatile Compounds in Reconstituted Milk Reduced-Fat Cheese and the Physicochemical Properties as Affected by Exopolysaccharide-Producing Strain. Molecules. 2012; 17(12):14393-14408. https://doi.org/10.3390/molecules171214393
Chicago/Turabian StyleWang, Weijun, Lanwei Zhang, and Yanhua Li. 2012. "Production of Volatile Compounds in Reconstituted Milk Reduced-Fat Cheese and the Physicochemical Properties as Affected by Exopolysaccharide-Producing Strain" Molecules 17, no. 12: 14393-14408. https://doi.org/10.3390/molecules171214393




